If you're an FSQA professional, 2025 was a year you won't soon forget. From record-breaking recalls to persistent operational challenges to unexpected wins, it was a year that tested the industry—and revealed what's working and what's not.
Here are the 10 most important trends, challenges, and insights that defined food safety and quality assurance in 2025.
1. Recalls Hit a Sobering Milestone
.avif)
Based on Q1-Q3 data, 2025 was on track for approximately 415 total recalls. To put that in perspective, that's more than one recall every single day somewhere in the U.S. food system.
The most common culprits? Undeclared allergens continued to dominate the recall landscape, along with Listeria contamination and foreign material contamination. For FSQA teams, this meant the margin for error kept shrinking. One mislabeled ingredient, one missed sanitation check, one piece of plastic from worn equipment—any of these could trigger a recall that costs millions and damages consumer trust built over decades.
2. Foodborne Illness Outbreaks Remained a Serious Threat
.avif)
The human impact of food safety failures became clear through the numbers:
- 33 multistate foodborne illness outbreaks affected communities across the country
- 921 confirmed patients suffered from preventable contamination incidents
Salmonella and Listeria continued to be the primary troublemakers, with leafy greens, poultry, and deli meats being frequent vectors. The CDC estimates that for every confirmed case, there are likely 20-30 more people who got sick but never reported it or got tested. So those 921 confirmed cases? They probably represent closer to 20,000 actual illnesses.
That's someone's grandmother, someone's kid, someone who just wanted to make dinner and ended up in the hospital instead.
3. FSQA Teams Carried an Enormous Workload
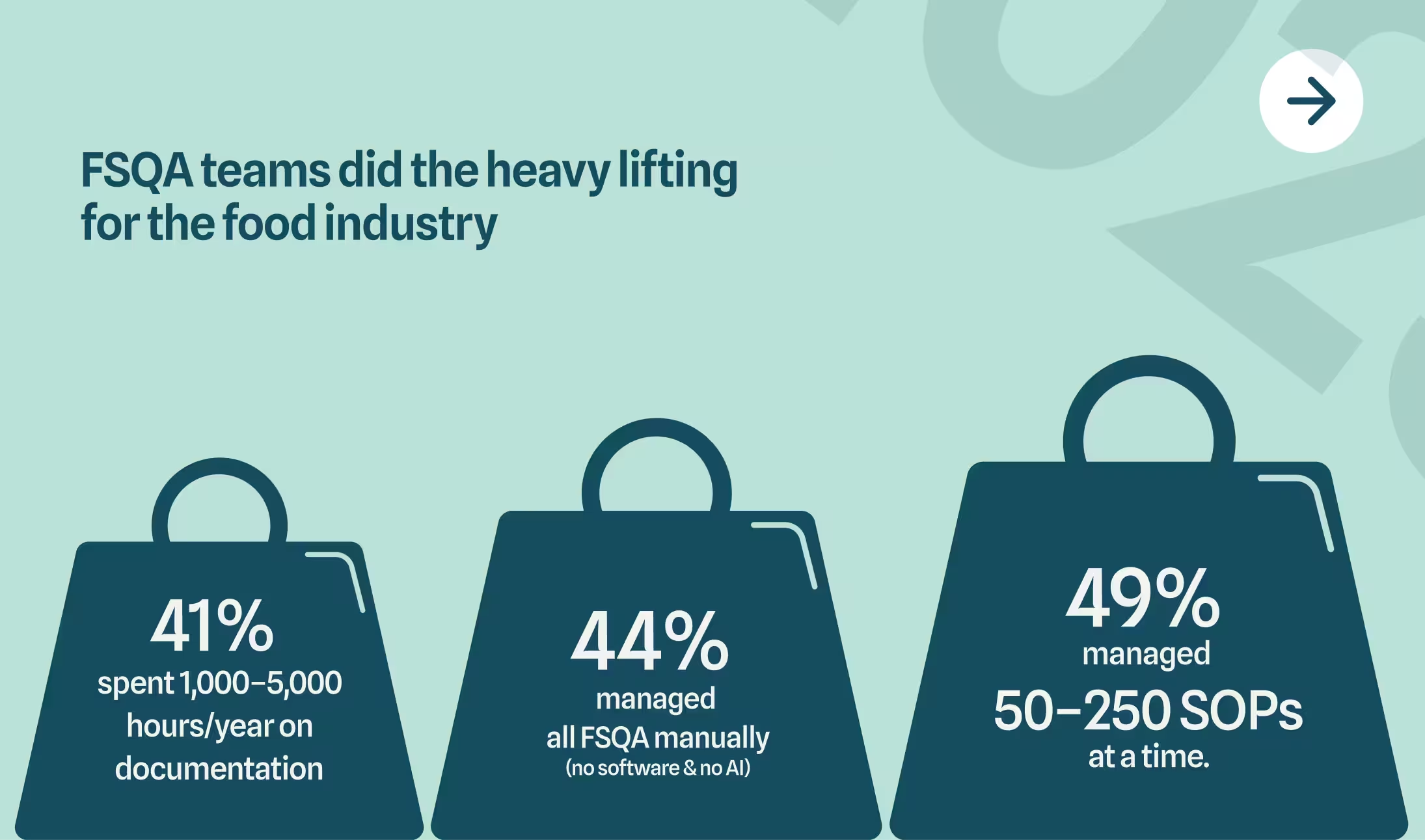
If you felt overwhelmed this year, the data confirms you weren't alone. Here's what FSQA teams were actually managing:
- 41% of teams spent between 1,000-5,000 hours per year just on documentation—the equivalent of multiple full-time employees doing nothing but paperwork
- 44% managed all FSQA processes manually, with no software or AI assistance to help streamline workflows
- 49% juggled between 50-250 Standard Operating Procedures simultaneously, each requiring regular review, updates, and compliance verification
And this is happening while you're also managing supplier approvals, investigating customer complaints, preparing for audits, training new staff, updating HACCP plans, and actually walking the production floor to catch problems before they become recalls.
The reality is that FSQA teams have become the backbone of food manufacturing, but the tools many are working with haven't evolved to match the complexity of the job.
4. Paper-Based Systems Caused Major Bottlenecks
.avif)
Despite best efforts, outdated systems created serious operational problems throughout the year:
- 18% of teams experienced lost paperwork that blocked product releases entirely
- 18% dealt with stuck or delayed product shipments
- 36% struggled to track down documents when mock recalls and audits demanded immediate answers
- 45% wasted 10-12 hours per week on manual record review
- 45% battled back-and-forth inefficiency just to get basic documentation completed
When you're racing against the clock during a potential recall situation, the difference between finding a document in 30 seconds versus 30 minutes can be the difference between a close call and a full-blown crisis.
The FDA expects you to be able to trace product within hours, not days, but paper filing systems weren't designed for that kind of speed.
5. The One Thing that Held Teams Back
.avif)
Despite all the progress in food safety science, technology, and training, one obstacle kept coming up in conversations with FSQA professionals: their documentation systems.
It's frustrating because the irony is obvious. The FDA requires extensive documentation to prove your food is safe. GFSI standards demand detailed records. Customers want audit trails. But when the system you use to manage all that documentation actively makes your job harder, something's broken. You end up spending more time managing the paperwork than actually preventing the problems the paperwork is supposed to track.
6. Most Quality Systems Were Still Outdated
.avif)
Perhaps the most eye-opening statistic of the year: 86% of FSQA teams reported their Quality Management System was still paper-based or overly clunky.
Think about that for a second. In 2025, when consumers can order food with a tap and track deliveries in real-time, most food safety teams are still using three-ring binders, Excel spreadsheets, and shared drives that nobody can find anything in.
Part of the problem is that food manufacturing often runs on tight margins, so investing in new systems feels impossible. Part of it is that ERP systems and generic quality software weren't built with food manufacturing in mind—they don't understand the difference between a HACCP deviation and a quality defect, or why allergen control needs to be handled differently than any other ingredient.
But the biggest reason might be simpler: "this is how we've always done it" is a powerful force, especially in an industry where change can feel risky.
7. The Core Problems Remained Consistent
.avif)
Throughout 2025, three persistent problems kept showing up:
- Slow product releases that kept innovation stuck in bureaucracy and delayed time-to-market
- Hours of manual work consuming time that should have been spent on actual quality improvements and risk prevention
- Lost paperwork creating blind spots in critical compliance processes and audit trails
The slow product release issue is particularly painful because it affects competitiveness. When your competitor can get a new product to market in 6 weeks but your FSQA approval process alone takes 4 weeks because paperwork keeps getting stuck between departments, you're losing business to bureaucracy, not to inferior quality.
And the lost paperwork problem? It's not usually that documents are actually lost forever. They're just lost temporarily—filed in the wrong place, saved under the wrong name, sitting on someone's desk waiting for a signature. But "temporarily lost" during an FDA inspection feels pretty permanent.
8. Teams Made Strategic Shifts in Their Priorities
.avif)
Despite the challenges, many FSQA teams took proactive steps to strengthen their operations:
- 38% committed to eliminating endless documentation cycles, recognizing that administrative burden was their biggest barrier to excellence
- 66% prioritized strengthening their food safety culture, understanding that technology alone wasn't enough—they needed their entire organization aligned around quality and safety
- 79% maintained zero tolerance for foreign materials, using better systems to track and prevent contamination with unprecedented precision
That 66% focusing on culture is particularly significant. You can have the best HACCP plan in the world, but if production supervisors see food safety as "the quality department's problem" or if operators don't feel empowered to stop the line when something's wrong, your plan is just paper.
The most successful facilities are the ones where everyone from the C-suite to the sanitation crew understands that food safety is everyone's job, and where people feel safe speaking up when they spot a problem.
9. Teams That Changed Their Approach Saw Real Results
.avif)
The teams that made strategic changes didn't just survive 2025—they actually improved:
- 46% caught problems early through routine inspections, before they became crises or customer complaints
- 92% made document control non-negotiable, establishing ironclad systems that ensured the right procedures were always accessible and up-to-date
- 36% strengthened supplier relationships by moving beyond simple auditing to genuine partnership and collaboration
That 46% early detection rate is huge. Finding a metal detector failure during your daily checks versus finding it because a customer bit into a piece of wire—same problem, vastly different outcomes. One is a near-miss you can learn from. The other is a recall, an FDA inspection, and potentially a lawsuit.
The supplier relationship piece is interesting too. The old model was adversarial: you audit them, find problems, and demand corrective actions. The new model recognizes that your suppliers' success is your success. When you work together to solve problems—sharing best practices, helping them understand your requirements, building actual relationships—everyone's food gets safer.
10. The Choice for 2026 Became Clear
.png)
By the end of 2025, the path forward had crystallized. You can keep fighting the same battles—spending 10-12 hours a week hunting for documents, manually reviewing records that could be automated, hoping nothing goes missing during your next audit.
Or you can look at what the teams who succeeded in 2025 did differently and ask yourself: what if we tried something new?
It doesn't have to be a massive overhaul. Sometimes it starts with digitizing one process, or piloting a new system in one facility, or just finally admitting out loud that the current way isn't working.
What 2025 Taught Us
Looking back at this year, a few things stand out:
The stakes are real. Over 400 recalls and nearly 1,000 confirmed illnesses aren't just statistics—they represent real failures with real consequences. Every hour you spend wrestling with paperwork is an hour you're not spending on prevention.
The workload isn't going to decrease. If anything, regulatory requirements keep expanding, customer expectations keep rising, and supply chains keep getting more complex. Hoping you can just "work harder" to keep up isn't sustainable.
Small changes can create real improvements. The teams that saw success in 2025 didn't necessarily make huge investments or overhaul everything at once. They made strategic choices about where to focus and were willing to try new approaches.
Culture eats strategy for breakfast. You can have the best systems in the world, but if your organization doesn't genuinely prioritize food safety, if people are afraid to report problems, if quality is seen as "someone else's job," you're building on sand.
Moving Forward
As you look ahead to 2026, start by asking yourself honest questions about your current systems:
- How many hours per week does your team spend on manual documentation?
- How quickly can you retrieve specific records during a mock recall?
- How often do documents get lost or misplaced?
- How much time elapses between identifying a problem and completing the corrective action paperwork?
Your answers will tell you everything you need to know about whether it's time for a change.
2025 showed us both the challenges facing food safety and the potential for improvement when teams are willing to evolve. What you do with those lessons in 2026 is up to you.
About This Data
The statistics and insights in this recap come from FSQA professionals across the food manufacturing industry who shared their experiences, challenges, and successes throughout 2025. Foodborne illness and recall data is based on FDA and CDC outbreak reports.
At Allera, we help food manufacturers modernize their FSQA operations with software designed specifically for the unique challenges of food safety and quality management. If you're interested in learning more about what modern FSQA systems can do for your team, we'd be happy to show you what's possible.
.avif)










.avif)

.avif)


.avif)
.avif)