

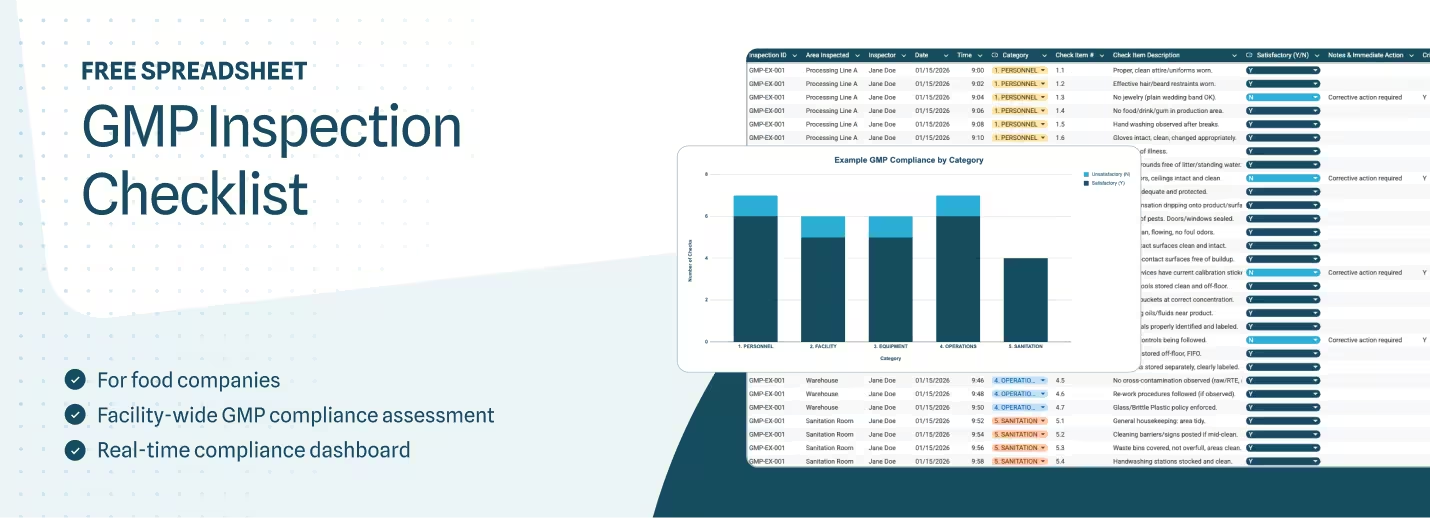
GMP Audit Checklist for Food Manufacturers (Free Template)
Good Manufacturing Practices audits only reduce regulatory risk when they actually verify what's happening on the floor.
This GMP audit checklist gives you a floor-level verification structure built for food manufacturers like you, with inspection items organized across five critical categories, action tracking that separates immediate corrections from systemic CAPAs, and a dashboard that shows compliance patterns by category.
Use it to verify personnel practices, facility conditions, equipment sanitation, operational controls, and sanitation execution before FDA walks your floor.
Plus you get critical finding flags and action owner assignments that force accountability for non-conformances that could trigger regulatory findings.
Keep reading to learn:
- How to use a floor-level GMP audit checklist
- What to verify across the five GMP categories during real walkthroughs
- How to document observations in real time so your records are defensible
- How to flag critical findings vs. minor issues and respond appropriately
- How to assign action owners, due dates, and link issues to CAPAs when needed
- How to use the dashboard to spot repeat failures and prioritize fixes
What's Included in Our GMP Audit Checklist?
Overview of the Spreadsheet Tabs
This GMP audit checklist includes four tabs:
- Instructions: How to use the template for floor inspections
- Audit Info: Facility details, inspection type, areas covered, and shift information
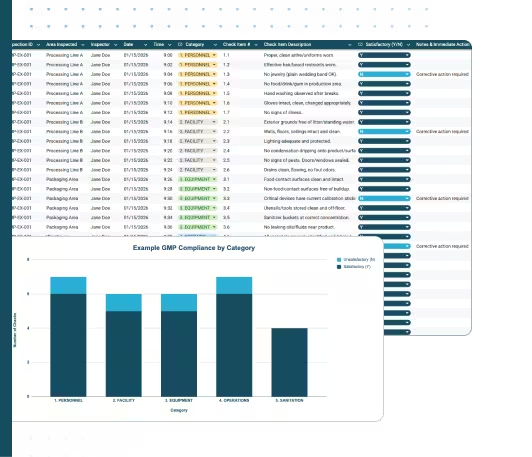
- GMP Inspection Checklist: Checkpoint items across five categories with Y/N scoring and action tracking
- Dashboard: Live compliance summary showing satisfactory vs. unsatisfactory findings by category
Each tab serves a specific function in creating a defensible GMP inspection record. The structure captures what FDA inspectors, third-party auditors, and GFSI certification bodies look for during facility walkthroughs.
How to Use Our GMP Audit Checklist
Now that you know what's included in our GMP audit checklist, it's time to dive deeper into each tab and understand how to use it for your internal inspections.
We'll start with the instructions tab:
Instructions
The Instructions tab defines the workflow for using this GMP audit checklist. It clarifies:
- Start in the 'GMP Inspection Checklist' tab
- Fill in Row 2 with Inspection ID, Area, Inspector, Date, and Time
- Drag those cells down to auto-populate the rest of the inspection
- Walk the floor and mark Y (Satisfactory) or N (Unsatisfactory) for each item
- Add notes and immediate actions where needed
- Review the Dashboard tab for live compliance results by category
This workflow enforces a critical discipline: you document as you walk, not after. GMP inspections lose value when observations are recorded from memory hours later. Real-time documentation captures what you actually saw, not what you think you saw.
The example tabs show a completed inspection (ID: GMP-EX-001) conducted by Jane Doe across four areas: Processing Line A, Processing Line B, Packaging Area, Warehouse, and Sanitation Room. Use these examples to understand how fields should be populated and how findings flow into the dashboard.
Audit Info
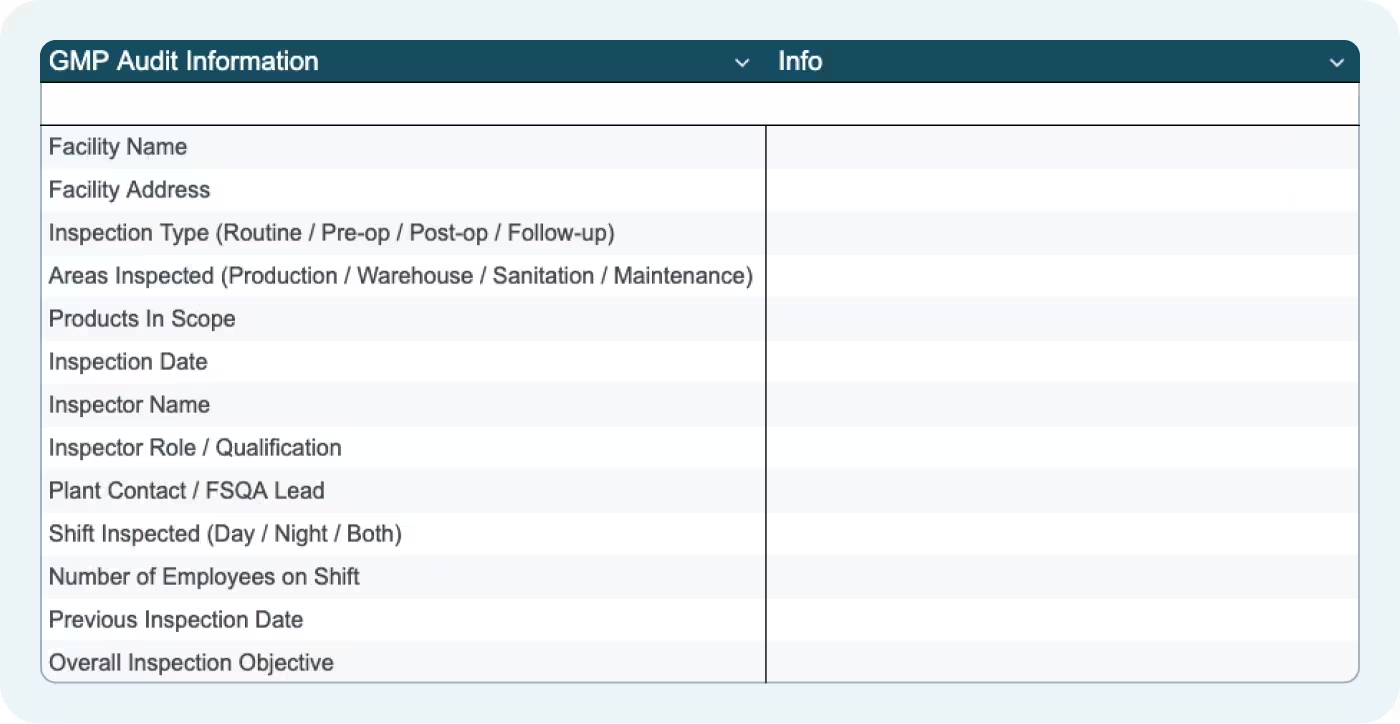
The Audit Info tab captures essential information that defines inspection scope and context:
- Facility Name and Address
- Inspection Type: Routine, Pre-op, Post-op, or Follow-up
- Areas Inspected: Production, Warehouse, Sanitation, Maintenance
- Products In Scope: Which product lines or allergen categories are being verified
- Inspection Date and Inspector Name
- Inspector Role/Qualification: QA Manager, Production Supervisor, FSQA Lead
- Plant Contact/FSQA Lead
- Shift Inspected: Day, Night, or Both
- Number of Employees on Shift
- Previous Inspection Date
- Overall Inspection Objective
This information creates a defensible inspection record. If an FDA investigator or third-party auditor asks "Who conducted this GMP inspection?" or "What shift did you verify?", the answer is documented here.
Inspection type matters. Routine inspections verify ongoing compliance. Pre-op inspections confirm sanitation before production starts. Post-op inspections verify cleaning after production. Follow-up inspections close out previous findings. Documenting inspection type prevents confusion about what was in scope.
Shift coverage is critical for 24-hour operations. If you only inspect day shift, you don't know what's happening at night. FDA investigators frequently ask whether GMP verification covers all shifts. Document which shift you inspected and justify gaps if they exist.
GMP Inspection Checklist
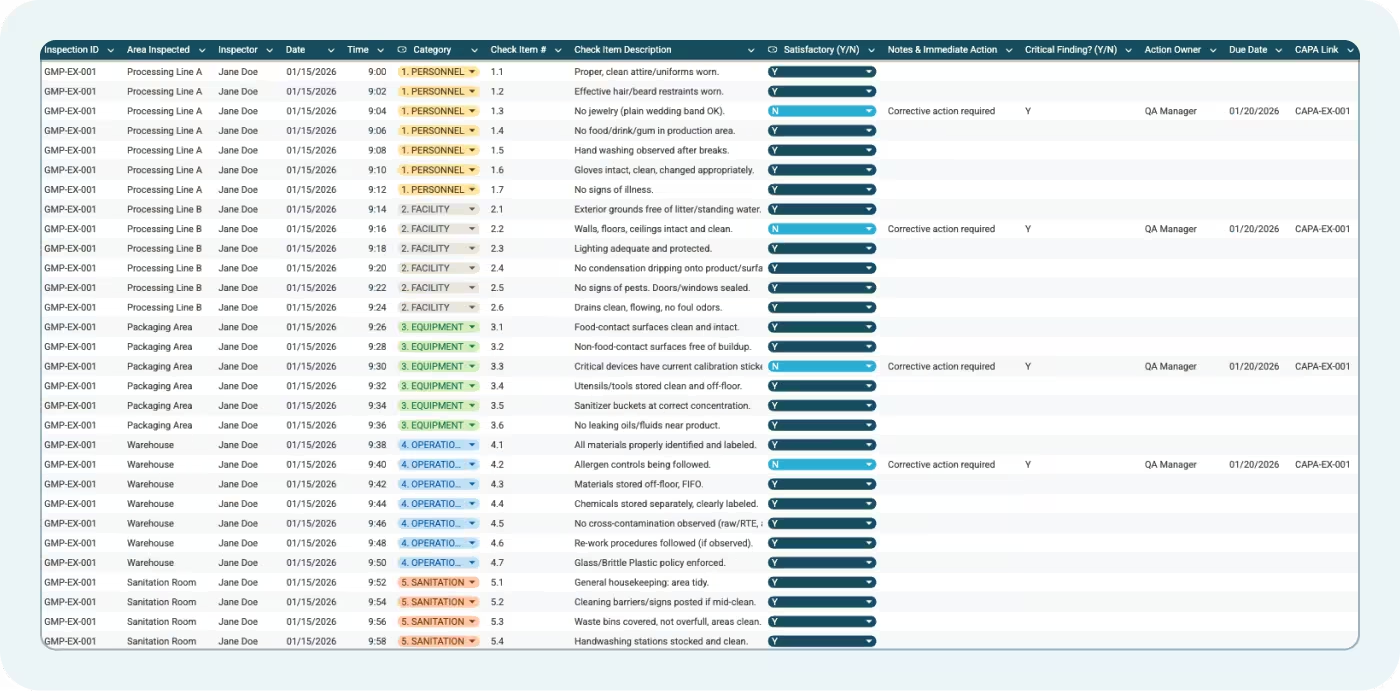
The GMP Inspection Checklist tab includes checkpoint items organized into five categories. Each row includes:
- Inspection ID: Unique identifier for traceability
- Area Inspected: Specific location (Processing Line A, Warehouse, Packaging Area)
- Inspector: Person conducting the verification
- Date and Time: When the observation occurred
- Category: Which GMP category the item falls under
- Check Item #: Numbered reference for each requirement
- Check Item Description: What you're verifying
- Satisfactory (Y/N): Dropdown for Yes or No
- Notes & Immediate Action: Space for observations and on-the-spot corrections
- Critical Finding? (Y/N): Flag for findings that pose immediate food safety or regulatory risk
- Action Owner: Person responsible for corrective action
- Due Date: When the action must be completed
- CAPA Link: Reference to formal corrective action record if needed
This structure separates observation (what you saw) from action (what needs to happen). That distinction matters because FDA expects you to demonstrate not just that you found problems, but that you fixed them and prevented recurrence.
The Five GMP Categories: What Each Checklist Item Actually Verifies
The checklist organizes 24 inspection items into five categories that cover the most common GMP failures FDA finds during facility inspections. Here's what you're actually verifying when you walk the floor:
1. Personnel (7 Items) - Verifying Human Hygiene Practices in Real Time
What you're documenting: Whether employees are following basic hygiene requirements that prevent contamination from people.
Items 1.1-1.3 (Attire, Hair Restraints, Jewelry): When you walk through production, you're looking at what employees are actually wearing. Is the QA technician wearing a hairnet that covers all their hair, or is there visible hair showing at the neckline? Is the production supervisor wearing their wedding ring, or are they wearing additional bracelets or watches? Document what you see, not what you assume happens when you're not there.
Item 1.4 (No Food/Drink/Gum): This is a visual sweep of work surfaces and employee pockets. Are there coffee cups on packing tables? Is someone chewing gum while handling product? These violations create direct contamination pathways.
Item 1.5 (Hand Washing After Breaks): This requires you to observe actual hand washing, not just check that sinks exist. Stand near the entrance after break time and watch whether employees wash for 20 seconds before re-entering production. If you mark this "Y" without observing actual hand washing, you're guessing.
Items 1.6-1.7 (Gloves, Illness): Are gloves torn or visibly dirty? Do you see employees touching their face and then touching food without changing gloves? Are there employees coughing or showing signs of illness in production areas? These are immediate contamination risks that need documentation and action.
2. Facility (6 Items) - Verifying Your Building Doesn't Contaminate Product
What you're documenting: Physical conditions of the facility that could introduce hazards.
Item 2.1 (Exterior Grounds): Walk outside before you walk inside. Is there standing water near receiving doors that could harbor bacteria or attract pests? Is trash piled against the building? External conditions migrate inside.
Item 2.2 (Walls, Floors, Ceilings): Look up, look down, look around. Is there peeling paint above a mixing station? Are there cracks in the floor where water and product accumulate? Is there visible rust on ceiling panels? Document the location—"ceiling damage above Line 3, Station 2" enables action. "Ceiling needs work" doesn't.
Item 2.3 (Lighting): Can you see well enough to spot contamination? Are there unprotected bulbs over exposed product? Broken light shields are critical findings because glass contamination is a recall hazard.
Item 2.4 (Condensation): The most critical facility item. Look at ceiling vents, pipes, and cooling units during production when temperature differentials create condensation. If you see drips forming above exposed product, that's an immediate stop-production finding.
Items 2.5-2.6 (Pests, Drains): Are door seals intact or can you see daylight under them? Are there gaps around pipes where pests could enter? Do drains smell or have visible buildup? These are evidence of pest pathways and sanitation failures.
3. Equipment (6 Items) - Verifying Cleaning and Maintenance Controls
What you're documenting: Whether equipment is maintained in sanitary condition and monitoring tools are reliable.
Items 3.1-3.2 (Surface Cleanliness): Run your hand along food-contact surfaces after cleaning. Is there sticky residue on conveyor belts? Is there product buildup in mixer corners? Now look at structural surfaces—are there grease drips on motor housings or ingredient dust on overhead beams? Both matter, but food-contact failures are critical findings.
Item 3.3 (Calibration): This is a sticker check. Are thermometers labeled with calibration due dates? Are those dates current? If a critical monitoring device (metal detector, thermometer, pH meter) has an expired calibration sticker, mark it critical and remove it from service.
Item 3.4 (Tool Storage): Are scoops and utensils stored off the floor on designated racks? Or are they sitting in buckets on the floor or leaning against walls? Storage violations are contamination risks and common FDA observations.
Item 3.5 (Sanitizer Concentration): Test sanitizer buckets with test strips during your inspection. Are they at the required concentration per your sanitation SOP? Buckets get diluted throughout shifts—verifying concentration during production tells you whether the system is working.
Item 3.6 (Leaking Fluids): Look under equipment for hydraulic fluid, oil, or grease near product zones. Leaking equipment introduces chemical contamination hazards and requires immediate maintenance.
4. Operations (7 Items) - Verifying Process Controls and Segregation
What you're documenting: Whether operational controls that prevent cross-contamination and mix-ups are actually being followed.
Item 4.1 (Material Identification): Are all ingredients, WIP (work in progress), and rework containers labeled with product name, lot code, and date? Unlabeled materials create allergen cross-contact and traceability failures.
Item 4.2 (Allergen Controls): If your facility handles allergens, verify physical segregation. Are allergen ingredients stored separately? Are dedicated utensils color-coded and used correctly? Are allergen production schedules being followed? This single item prevents most allergen recalls.
Item 4.3 (Storage and FIFO): Walk the warehouse. Are pallets stored directly on the floor, or are they on pallets/racks? Is FIFO actually happening, or are older lots pushed to the back? Check lot codes to verify rotation.
Item 4.4 (Chemical Storage): Are cleaning chemicals and sanitizers stored in a locked room separate from ingredients? Are spray bottles labeled? Unlabeled spray bottles near food are automatic FDA observations.
Item 4.5 (Cross-Contamination): The observational item. Are raw materials physically separated from RTE products? Do employees move between allergen and non-allergen zones without changing gloves? Are tools shared between raw and cooked areas? Document what you see happening during production.
Items 4.6-4.7 (Rework, Glass/Brittle Plastic): If rework is happening during your inspection, verify procedures are followed (segregation, labeling, approved usage). Check for glass or brittle plastic in production zones—drinking glasses, personal phones, unauthorized clipboards with plastic covers. These are foreign material hazards.
5. Sanitation (4 Items) - Verifying Daily Cleaning Execution
What you're documenting: Whether sanitation happens between procedures and observations.
Item 5.1 (Housekeeping): Is the production area cluttered or organized? Are empty pallets stacked neatly or scattered? Is there product residue on floors from the previous shift? Housekeeping reveals whether sanitation is continuous or just pre-audit theater.
Item 5.2 (Cleaning Barriers): If you walk through during mid-shift cleaning, are "cleaning in progress" signs posted? Are barriers set up to prevent personnel from entering partially cleaned zones? This verifies your sanitation procedures are being followed.
Item 5.3 (Waste Management): Are trash bins covered and emptied before overflowing? Is the area around waste bins clean, or is there trash on the floor? Waste management failures attract pests and create sanitation violations.
Item 5.4 (Hand Washing Stations): Are sinks stocked with soap, paper towels, and hand sanitizer? Are they clean, or are they covered in splatter and residue? A non-functional hand washing station means employees can't follow hygiene requirements even if they want to.
GMP Audit Dashboard
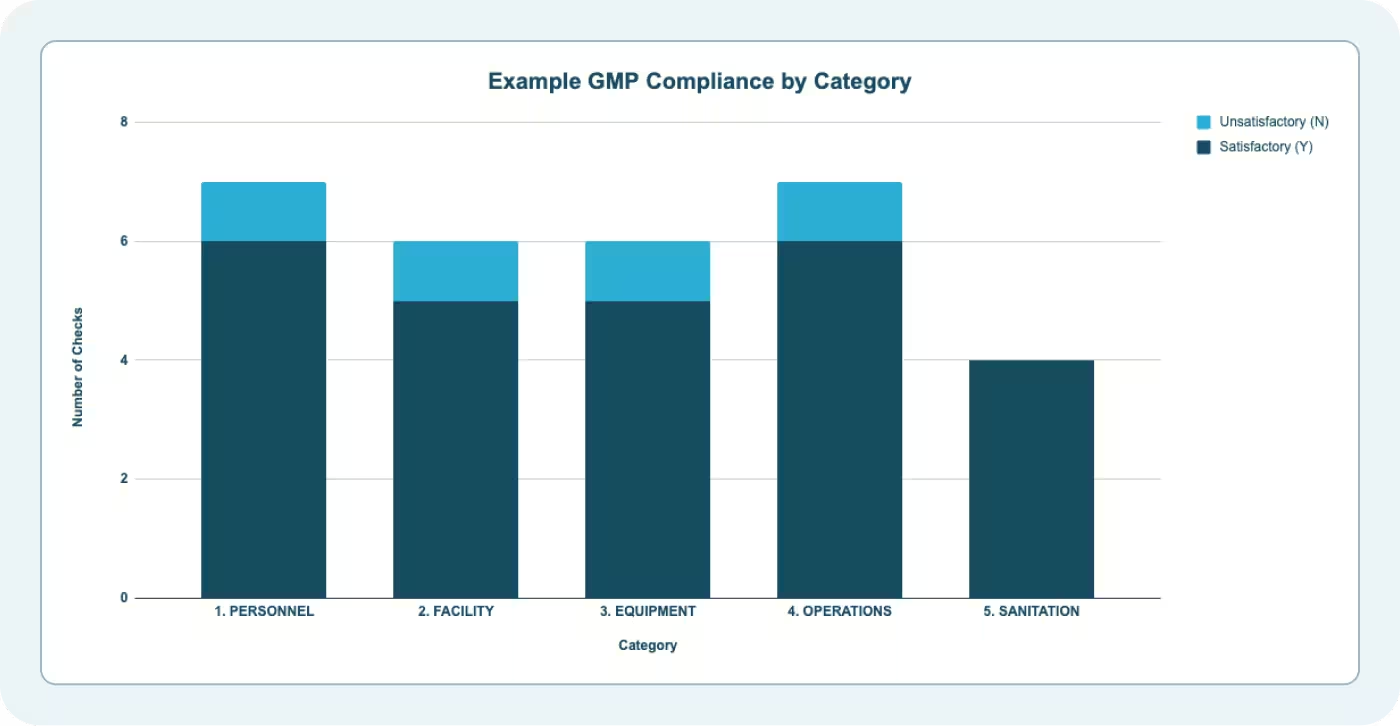
The Dashboard tab summarizes GMP inspection results by category:
- Satisfactory (Y) count per category
- Unsatisfactory (N) count per category
- Total compliance by category
This summary feeds management review and helps food safety professionals answer critical questions:
- Which GMP categories have the most findings?
- Are we improving or regressing between inspections?
- Do we have isolated lapses or systemic issues?
If three consecutive inspections show unsatisfactory findings in Category 1 (Personnel), that's a systemic issue. Management review should include a decision to retrain employees, increase supervision, or revise hiring standards.
If findings are scattered across different categories each inspection, you likely have an execution issue—supervision gaps, unclear expectations, or resource constraints.
Use the dashboard to set priorities for corrective action. Categories with repeat findings need deeper investigation. Categories with critical findings need immediate action.
Why GMP Audits Matter in the Food Industry
Good Manufacturing Practices are the foundation of food safety compliance. FDA enforces GMPs through 21 CFR Part 117 (Preventive Controls for Human Food) and uses GMP deficiencies as the basis for warning letters, import alerts, and consent decrees.
GMP violations are among the most frequently cited issues in FDA Form 483s issued to food manufacturers. The most common citations include:
- Failure to maintain facilities in a sanitary condition
- Employees not following personnel hygiene requirements
- Equipment not properly cleaned and sanitized
- Pest activity observed in production areas
- Condensate dripping onto exposed product
These aren't theoretical risks—they're the findings that trigger regulatory action. Internal GMP audits reduce that risk by finding and fixing problems before FDA inspectors arrive.
GMP Requirements Under FSMA and FDA Regulations
The Food Safety Modernization Act (FSMA) shifted FDA's enforcement focus from reactive responses to preventive controls. But GMPs remain the baseline. You can't have effective preventive controls if your facility doesn't meet basic GMP requirements.
21 CFR 117 Subpart B defines current good manufacturing practices for food facilities. Key requirements include:
- Personnel must maintain personal cleanliness and use hygienic practices
- Plant and grounds must be maintained to prevent contamination
- Sanitary operations must prevent contamination during food handling
- Equipment and utensils must be designed and maintained for sanitary conditions
- Processes and controls must be monitored to ensure food is safe
These regulations aren't new, but FSMA increased enforcement. FDA investigators now look for evidence that you're verifying GMP compliance, not just documenting procedures.
GMP Expectations Under GFSI Schemes
Global Food Safety Initiative (GFSI) schemes like SQF, BRCGS, and FSSC 22000 all include GMP requirements. These standards expect facilities to demonstrate:
- Documented GMP procedures
- Evidence of GMP verification (inspections, audits, monitoring)
- Corrective action when GMP failures occur
- Management review of GMP performance
GFSI auditors classify non-conformances by severity (e.g., minor, major, or critical) based on food safety impact, with terminology varying by scheme. Repeat GMP findings or systemic GMP breakdowns can prevent certification or trigger recertification audits.
Internal GMP audits help facilities prepare for GFSI certification by identifying gaps before the auditor does. If your checklist mirrors what GFSI auditors verify, you're practicing the same verification the auditor will perform.
Common GMP Audit Failures the Checklist Helps Prevent
Most GMP inspections fail not because facilities lack procedures, but because verification doesn't match what regulators and auditors expect. Here are the most common gaps this GMP audit checklist prevents:
Observations Without Documentation
The most common GMP inspection failure: you walked the floor, you saw things, but you didn't write them down in a way that creates a defensible record.
Example: You observe employees on the production floor and mentally note that hand washing looks good. But when FDA asks for evidence of GMP verification, you have no written record of what you observed, when you observed it, or who was on shift.
This checklist prevents that by forcing you to document:
- Inspection ID and date
- Specific area inspected
- Who conducted the inspection
- What time the observation occurred
- Whether each item was satisfactory or unsatisfactory
If you can't point to a record that says "Inspected Processing Line A on 01/15/2026 at 9:00 AM, observed hand washing after breaks, marked satisfactory," you don't have verification—you have an unsubstantiated claim.
No Critical Finding Flags
Another common failure: treating all GMP findings the same, regardless of food safety impact.
Example: You find a minor scuff on the floor and uncalibrated thermometers on the same inspection. Both get marked as "needs attention," but one is cosmetic and one is a critical control failure.
This checklist includes a Critical Finding? (Y/N) field that forces you to differentiate between immediate food safety risks and minor lapses. Critical findings should trigger:
- Immediate corrective action (stop production if necessary)
- Management notification
- Formal CAPA documentation
- Verification that the issue is resolved before resuming operations
If your GMP checklist doesn't flag critical findings, you can't prioritize action appropriately.
No Action Owner or Due Date
The third common failure: you document findings, but no one owns the corrective action and there's no deadline for closure.
Example: You note "Walls need repair in packaging area" during a GMP inspection. Three months later, the walls still aren't repaired. When the auditor asks why, there's no record of who was responsible or when the repair was supposed to happen.
This checklist includes Action Owner and Due Date fields for every unsatisfactory finding. If you mark something as "N," you must assign responsibility and set a deadline. That creates accountability and enables follow-up verification.
Inspection Scope Gaps
The fourth common failure: you inspect the same areas during the same shifts and miss high-risk zones or off-hours conditions.
Example: You conduct GMP inspections every Monday at 10 AM on day shift. Night shift operates differently—different supervisors, different employees, different cleaning crews. You don't know what's happening at night, and neither does management.
This checklist's Audit Info tab forces you to document:
- Areas inspected (Production, Warehouse, Sanitation, Maintenance)
- Shift inspected (Day, Night, Both)
- Number of employees on shift
When you review inspection records over time, you can identify coverage gaps. If you've never inspected night shift, that's a risk. If you've never inspected the maintenance shop, that's a blind spot.
No Trend Analysis
The fifth common failure: you conduct inspections, but you don't analyze results to identify patterns.
Example: You find hand washing violations on three consecutive inspections. Each time, you retrain the individual employee. But the root cause isn't individual performance—it's that hand washing stations are too far from the production entrance, so employees skip it when they're in a hurry.
The Dashboard tab aggregates findings by category. If Category 1 (Personnel) consistently shows unsatisfactory findings, that's a trend. Management review should investigate whether the issue is training, supervision, facility design, or procedure clarity.
Trend analysis turns inspection data into actionable intelligence. Without it, you're just collecting records.
What is a GMP Audit Checklist?
GMP Inspection vs. GMP Audit vs. GMP Verification
The terms "inspection," "audit," and "verification" are often used interchangeably, but they have different meanings in food safety:
GMP Inspection is a physical walkthrough of the facility to verify current conditions. You're observing personnel practices, facility status, and operational execution in real time. GMP inspections are typically conducted daily, weekly, or shift-by-shift.
GMP Audit is a systematic review of procedures, records, and implementation to verify conformance against a standard (FDA regulations, GFSI requirements, company policies). Audits are typically conducted annually or semi-annually and include documentation review alongside floor observations.
GMP Verification is the umbrella term for any activity that confirms GMP requirements are being met. This includes inspections, audits, monitoring, and testing.
This GMP checklist is designed for inspections—frequent, floor-level verification that captures current conditions. It's lighter than a full audit (which would include procedure review and record traceability) but more structured than an informal walkthrough.
Why Generic GMP Checklists Fail in Food Manufacturing
Most free GMP checklists are designed for pharmaceutical manufacturing, not food. They include items like cleanroom classifications, environmental monitoring, and validation protocols that don't apply to food facilities.
Food-specific GMPs focus on:
- Personnel hygiene and health (21 CFR 117.10)
- Facility and grounds maintenance (21 CFR 117.20)
- Allergen control and cross-contamination prevention
- Pest control and sanitation execution
- FIFO, chemical storage, and waste management
Generic checklists also fail to capture floor-level execution. A checklist that asks "Is there a hand washing procedure?" doesn't verify whether employees are actually washing their hands. A checklist that asks "Are allergens segregated?" doesn't verify whether the segregation is effective on the production floor.
This GMP checklist focuses on what you can observe and verify during a facility walkthrough, not what's documented in procedures.
What FDA Inspectors Look for During GMP Verification
FDA investigators use the same observational approach this checklist follows. When they walk your facility, they're looking for:
- Employees wearing jewelry or improper attire
- Evidence of pests (droppings, gnaw marks, live insects)
- Condensation dripping onto product or food-contact surfaces
- Uncovered waste bins or overflowing trash
- Chemicals stored near food or inadequately labeled
- Equipment with visible residue or damage
- Hand washing stations without soap or paper towels
These are objective observations that can be documented in real time. FDA investigators write them in Form 483s because they're verifiable. Your internal GMP inspections should document the same observations before FDA finds them.
How to Use the GMP Audit Checklist for Internal Inspections
Frequency and Timing Recommendations
GMP inspection frequency depends on facility size, production complexity, and risk level. Common approaches:
Daily Pre-Op Inspections: Conducted before production starts to verify sanitation, pest control, and facility readiness. Use a subset of this checklist (categories 2, 3, and 5) to confirm the environment is ready for food production.
Weekly Routine Inspections: Conducted during production to verify personnel practices, operational controls, and ongoing sanitation. Use the full checklist and rotate areas to ensure comprehensive coverage over time.
Monthly Comprehensive Inspections: Conducted across all shifts and all areas to verify GMP compliance at the facility level. Use the full checklist and document trends in the dashboard.
Follow-Up Inspections: Conducted after corrective action to verify that findings are closed. Use the specific checklist items that were marked unsatisfactory to confirm improvement.
FDA doesn't mandate GMP inspection frequency, but FSMA requires you to demonstrate that preventive controls are effective. If your GMP inspections are infrequent or superficial, you can't demonstrate control.
Inspector Qualifications and Training
GMP inspections should be conducted by someone who understands:
- Regulatory requirements (21 CFR 117 and relevant GFSI standards)
- Food safety hazards (biological, chemical, physical, allergen)
- Facility-specific procedures and standards
- How to document observations objectively
Common inspectors include QA managers, FSQA leads, production supervisors, and trained quality technicians. The inspector doesn't need to be independent (unlike internal audits under SQF or BRCGS), but they do need to be competent and objective.
Train inspectors on:
- What each checklist item means and how to verify it
- How to differentiate between satisfactory and unsatisfactory
- When to flag a finding as critical
- How to document observations without bias or assumption
If your inspector writes "hand washing looks fine," that's not a defensible record. If they write "Observed 4 employees entering production area; all washed hands for 20 seconds per SOP before entering," that's verification.
How to Document Observations and Immediate Actions
The Notes & Immediate Action field is where you capture what you saw and what you did about it.
Satisfactory findings don't require extensive notes. A simple confirmation like "Observed employees wearing hair nets and beard restraints" is sufficient.
Unsatisfactory findings require specificity:
- What did you observe? ("Condensation dripping from ceiling vent onto packaging table")
- Where was it located? ("Processing Line B, Station 3")
- What immediate action was taken? ("Stopped production, notified maintenance, redirected condensate to drain")
Immediate actions are temporary fixes that prevent the issue from causing contamination while you investigate root cause. Immediate action is not corrective action—it's containment.
If you find expired ingredients during a GMP inspection, immediate action is removing them from inventory. Corrective action is updating the FIFO procedure and retraining receiving staff. Both should be documented, but in different places.
Linking GMP Findings to Formal CAPAs
Not every GMP finding requires a formal corrective action (CAPA). Minor, isolated lapses can be addressed with immediate correction and supervisor coaching.
But some findings do require formal CAPAs:
- Critical findings (immediate food safety or regulatory risk)
- Repeat findings (same issue found on multiple inspections)
- Systemic issues (patterns across multiple areas or shifts)
The CAPA Link field in this checklist is where you reference the formal CAPA record. If a GMP finding triggers CAPA-2025-042, document that link so you can trace from the inspection finding to the root cause analysis and preventive action.
This traceability matters during audits. If an auditor sees a GMP finding flagged as critical but no CAPA reference, they'll question whether your corrective action process is effective.
When to Transition from Spreadsheet to GMP Management Software
Spreadsheets work well for facilities with:
- Consistent inspection frequency (weekly or monthly)
- One or two inspectors
- Stable areas and inspection scope
- Low corrective action volume
Spreadsheets start to fail when:
- Multiple people need to conduct inspections simultaneously
- You're managing corrective actions across departments
- You need trend analysis across multiple months or years
- You have multiple facilities that need standardized GMP verification
Version Control and Multi-Inspector Challenges
The biggest spreadsheet limitation is version control. If three people are conducting GMP inspections using the same template, how do you consolidate results? If someone updates the checklist, how do you ensure everyone is using the current version?
Version control becomes a problem when:
- Inspections are conducted daily or multiple times per week
- Different shifts use different versions of the checklist
- The checklist is updated after an audit or regulatory change
If you're emailing spreadsheets back and forth or saving them to shared drives with file names like "GMP_Checklist_Final_v3_UPDATED.xlsx," you have a version control problem.
Evidence Traceability and CAPA Integration
This checklist includes a CAPA Link field, but the CAPA record lives somewhere else—probably in another spreadsheet, a quality management system, or a paper file.
When an auditor asks "Show me the CAPA for this finding," you have to manually locate the referenced CAPA. If the CAPA reference is wrong or incomplete, you can't trace from the finding to the corrective action.
Food traceability matters because it demonstrates:
- Findings are investigated, not ignored
- Root causes are identified, not assumed
- Preventive actions are implemented, not just discussed
- Effectiveness is verified, not just claimed
Spreadsheets can't link directly to evidence. You're documenting that a link exists, but the auditor has to trust that the link is accurate.
When GMP Inspections Need Real-Time Dashboards
The Dashboard tab in this spreadsheet updates when you enter data, but it doesn't aggregate findings across multiple inspections or show trends over time without manual work.
If you want to answer questions like:
- How many critical findings did we have last quarter?
- Which areas have the most repeat findings?
- Are GMP scores improving or declining month-over-month?
- How many overdue corrective actions do we have across all GMP inspections?
You need either significant manual analysis or a system that aggregates and visualizes data automatically. For facilities conducting GMP inspections weekly or daily, manual analysis becomes a bottleneck.
When spreadsheets create version control issues, evidence gaps, or trend analysis delays, that's when you need a system that links GMP inspections to CAPAs, procedures, and training records in real time. Systems like Allera's FSQA platform provide centralized GMP inspection tracking with automated dashboards, CAPA integration, and version-controlled checklists that update across all users simultaneously.
Download the Free GMP Audit Checklist
This GMP inspection checklist is designed for:
- QA managers and FSQA leads conducting routine GMP inspections
- Production supervisors verifying floor-level compliance
- Facilities preparing for FDA inspections or GFSI certification
- Operations teams that need a structured GMP verification tool without investing in software
To get the most value from this checklist:
- Customize the checklist items to match your facility's scope (add or remove items based on your operations)
- Train inspectors on how to document observations objectively and flag critical findings
- Use the Dashboard tab to identify trends and prioritize corrective action
- Link critical and repeat findings to formal CAPAs for root cause analysis and preventive action
- Review inspection results during management review to identify systemic issues
Spreadsheet limitations to be aware of:
- Version control depends on file naming and manual distribution
- Evidence traceability is limited to what's written in the Notes field
- No automated alerts for overdue actions
- Dashboard requires manual updates if you modify the checklist structure
- Trend analysis across multiple inspections requires manual consolidation
These limitations don't prevent effective GMP inspections—they just mean you need discipline around file management and follow-through.
Final Thoughts
Effective GMP inspections verify what's happening on the floor, not what's written in procedures. They document observations objectively, flag critical findings immediately, and create accountability for corrective action.
Use this checklist to enforce observation discipline, critical finding identification, and action owner assignment. Track trends. Close findings before regulators or auditors find them. Treat your GMP inspection program like the regulatory risk control it's supposed to be.
When spreadsheets create version control issues, evidence traceability gaps, or trend analysis delays, that's when you need a system that links GMP inspections to CAPAs, procedures, and training records in real time. But until then, this GMP audit checklist gives you a structured, floor-level verification tool for food manufacturing compliance.
Our team spent a lot of time on making this checklist the best it could be. If you found it helpful, we'd highly appreciate you sharing it with a friend in the food industry. Thank you from the Allera Team 💙
.avif)






.avif)


.avif)


.avif)