TLDR:
- GMP is now a proactive, end-to-end system that builds food safety and quality assurance into every stage of production.
- cGMP requires continuously updating programs to keep pace with changing regulations, technology, and emerging risks.
- Most GMP failures stem from recurring issues in personnel practices, facility conditions, process controls, and documentation.
- Strong GMP programs use risk-based assessments, CAPA, internal audits, and training to fix root causes, not just symptoms.
- Paper systems limit visibility and accountability, while digital GMP tools enable real-time data, trends, and faster decisions.
- Leading and lagging indicators together provide a fuller picture of GMP performance and continuous improvement.
- Allera helps food manufacturers centralize GMP, automate workflows, and turn compliance into a strategic advantage.
.avif)
Have you ever wondered what separates world-class food manufacturers from those constantly battling recalls and failed audits? The answer lies in three letters: GMP. As you navigate 2026's complex regulatory landscape, Good Manufacturing Practices have evolved beyond simple compliance into comprehensive systems defining operational excellence in your facility.
Whether you're an FSQA manager, director, supervisor, operator, or even a tech, this guide will help you better understand GMPs across your food company! We hope you enjoy!
Why GMP Matters in the Food Industry
Every decision you make in your facility directly impacts consumer safety. You're not just managing production lines—you're safeguarding public health and your business reputation.
The costs of GMP failures hit hard: millions in recall expenses, lost sales during and after recall events, legal liabilities and potential criminal penalties, brand damage requiring years to repair, and retailer delisting that eliminates distribution channels. One contamination event can destroy decades of trust.
But here's what you gain with GMP excellence:
- Dramatically fewer quality defects and customer complaints
- Reduced waste cutting into your profit margins
- Smoother operations with less downtime
- Stronger retailer relationships built on proven reliability
- Competitive advantage in quality-conscious markets
In today's marketplace, your GMP performance isn't just about avoiding disasters—it's your competitive edge that opens doors or closes them.
GMP Meaning in The Food Industry?
You need to understand GMP as your systematic framework ensuring products are consistently produced and controlled to quality standards. It's not a single procedure you can check off—it's woven into every operation aspect, from how you design your facility to how employees wash their hands.
Here's the fundamental shift in thinking: you're building quality into your processes, not trying to inspect it in afterward. When you embrace this proactive approach, you identify and control risks before they become crises that make headlines.
Your GMP framework encompasses raw material sourcing and supplier verification, facility design preventing contamination, equipment calibration and sanitation schedules, personnel hygiene and training protocols, production parameter monitoring, quality assurance checkpoints throughout processing, comprehensive documentation of all critical activities, and investigation procedures when deviations occur. Think of it as your integrated management system rather than isolated requirements.
What GMP and cGMP Mean for Food Manufacturers
GMP represents your baseline—the fundamental practices you must follow for safe food production. But you've probably also seen "cGMP" and wondered about the difference. That "c" stands for "current," and it changes everything about your approach.
You can't implement a program once and consider yourself done. The cGMP requirement means you're continuously evaluating practices against evolving industry standards, emerging food safety science, new regulatory expectations, and technological capabilities available to you.
Think about challenges your facility faces now that didn't exist five years ago: plant-based proteins with novel processing requirements, automation technologies changing contamination risk profiles, consumer transparency expectations around sourcing, emerging pathogens and antimicrobial resistance, and climate change impacts on ingredient safety. Your GMP program needs adapting to these realities, or you'll fall behind competitors who do.
When auditors evaluate your facility, they're checking whether you're implementing truly current practices or just following outdated procedures because "that's how we've always done it." You need evidence of regular program reviews, benchmarking against industry leaders, and willingness to invest in improvements when gaps surface.
Maintaining current GMP requires more than updating procedures periodically. It depends on a structured management approach that governs how GMP expectations are defined, carried out on the production floor, verified through records and audits, and improved when gaps are identified.
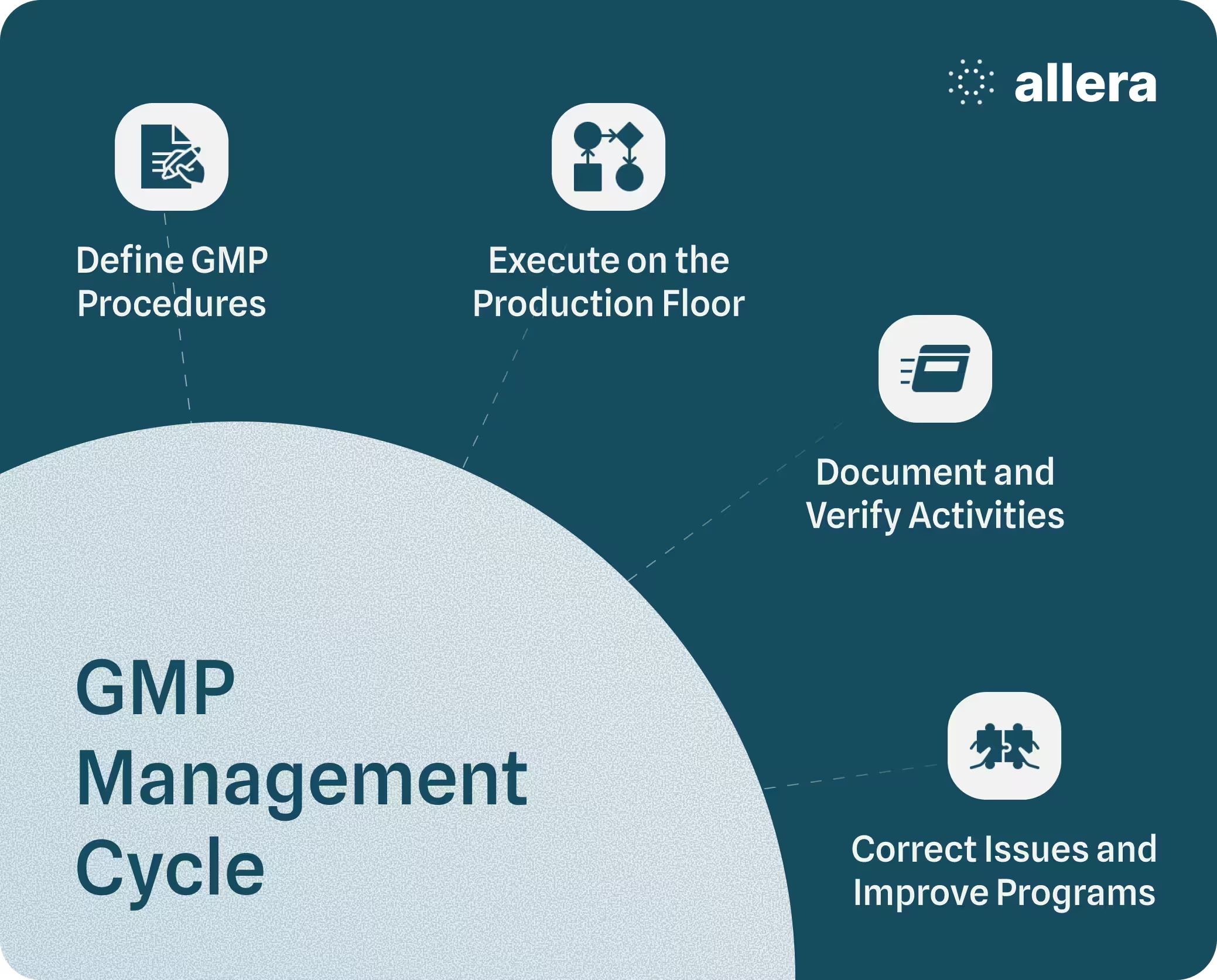
This management cycle applies across all areas of GMP, from personnel practices and facility conditions to process controls and documentation.
Core GMP Requirements in Food Manufacturing
While GMP encompasses broad territory, requirements generally fall into several core categories that you'll need to master.
.avif)
Personnel Requirements
Your people represent your first defense against contamination. You need everyone entering production areas to understand and consistently follow practices preventing food safety failures.
Health and hygiene standards you must enforce include illness reporting before employees enter production areas, proper handwashing at designated stations and critical moments, correct use of personal protective equipment including hairnets and gloves, restrictions on jewelry and items creating physical hazards, and clean outer garments worn only in production zones.
Training forms another critical pillar. You can't assume employees instinctively know proper practices. Every team member needs training appropriate to their responsibilities—and this isn't one-time orientation during onboarding. You're building ongoing education that reinforces practices, addresses identified gaps, and covers new procedures as your programs evolve.
Perhaps most challenging, you need cultivating a culture where employees genuinely understand why these practices matter and feel empowered to speak up when they spot problems. Rules alone don't create compliance—you need engagement and accountability.
Facility and Equipment Standards
Your facility's design and condition directly determine your ability to produce safe food. You can't manufacture clean products in dirty, poorly maintained environments.
Building requirements you must meet include construction suitable for intended use with food-grade materials, physical separation between raw and ready-to-eat processing areas, adequate lighting allowing staff to see potential contamination, proper ventilation preventing condensation, pest-proof design eliminating harboring areas and entry points, and floors, walls, and ceilings constructed from cleanable, non-absorbent materials.
Equipment standards demand your attention too. Design must be suitable for intended food contact applications, construction from materials that won't migrate harmful substances, installation allowing access for thorough cleaning and inspection, preventive maintenance programs stopping breakdowns before contamination occurs, and calibration schedules ensuring monitoring accuracy. Don't overlook utilities—your water supply, waste disposal systems, and compressed air all need meeting food safety specifications.
Production and Process Controls
This is where GMP meets your daily manufacturing reality. You need established procedures ensuring processes perform consistently and critical parameters stay within safe limits.
Production controls you must implement:
- Raw material receiving checks verifying supplier compliance
- Allergen management preventing cross-contact
- Time and temperature monitoring at critical steps
- In-process checks catching deviations before entire batches are affected
- Rework and reprocessing procedures preventing quality degradation
Process validation provides evidence that your procedures actually achieve intended outcomes—whether that's a specific pathogen kill step or proper ingredient dispersion. You can't just assume processes work; you need demonstrating they consistently deliver safe products.
Documentation and Record-Keeping
If you didn’t document it, you can’t prove it happened—which is why many food manufacturers centralize GMP records, SOPs, and revision histories using a digital document control system instead of binders and shared drives. That's the regulatory reality you face. GMP demands comprehensive documentation of your programs plus records demonstrating consistent execution.
You need written standard operating procedures for all GMP-related activities, batch production records showing what actually occurred during specific production runs, monitoring records for critical control points, equipment calibration and maintenance logs, personnel training records with dates and topics covered, deviation and investigation reports when problems arise, and corrective action documentation proving you closed gaps.
Your records need accuracy, completeness, and immediate retrievability. You must protect them from unauthorized alterations and maintain them for appropriate periods—often years based on product shelf life and regulatory requirements.
Common GMP Categories: People, Premises, Processes, Records
Many food safety professionals organize GMP using the "4 Ps" framework. This structure helps you ensure comprehensive program coverage without gaps.
People
Everything starts with your workforce. This category addresses employee health and hygiene, training and competency, visitor management, and building food safety culture that actually sticks.
The human element often determines where GMP succeeds or fails. An employee rushing through handwashing to meet production targets, coming to work sick due to attendance pressure, or taking undocumented shortcuts can undermine your best-designed systems.
Critical people factors you must manage include thorough onboarding covering facility-specific requirements, multilingual training materials matching your workforce, temporary worker protocols ensuring consistent standards, visitor and contractor controls preventing contamination introduction, and leadership behaviors modeling expected standards rather than just demanding them.
Ask yourself: do employees genuinely understand why handwashing matters, or do they view it as bureaucratic hassle? Are temporary workers receiving abbreviated training that creates gaps? Is there unspoken pressure to work when sick? These people-related issues require thoughtful program design and sustained leadership commitment.
Premises
Your facility and its condition create the environment where food safety either thrives or fails. This category covers design, layout, maintenance, sanitation, and pest management.
Smart facility design makes GMP easier to execute. Proper workflow prevents cross-contamination between raw and ready-to-eat areas. Adequate space allows thorough cleaning between production runs. Appropriate construction materials resist harboring pathogens.
Facility elements demanding your focus include zoning establishing clear boundaries between contamination risk levels, traffic patterns for people and materials minimizing cross-contamination, building envelope integrity preventing pest entry, utility systems designed for food-grade service, master sanitation schedules covering all areas and equipment, and preventive maintenance preventing deterioration before problems emerge.
Design alone doesn't guarantee success. You need vigilant maintenance preventing deteriorating conditions—like peeling paint above processing areas or leaking roofs introducing water that fosters pathogen growth.
Processes
This category addresses how you transform ingredients into finished products safely. It encompasses raw material management, production procedures, allergen controls, process monitoring, and product handling.
Your processes need design with food safety built in from the start, validation proving they work as intended, consistent execution following documented procedures, and real-time monitoring catching deviations before batches are completed. Process elements you must control include supplier approval programs verifying GMP compliance upstream, receiving procedures catching problems before materials enter your facility, storage conditions preventing quality degradation, production sequences minimizing allergen cross-contact risks, and hold and release systems preventing premature distribution.
Records
Documentation provides the evidence proving your GMP program exists beyond paper and actually functions daily. This category covers both your written procedures and the records demonstrating compliance.
Records serve critical functions: proving due diligence if regulatory or legal issues arise, enabling root cause analysis when problems occur, tracking trends revealing deteriorating performance before crises, supporting continuous improvement decisions with data, and demonstrating competence to customers and auditors.
You need balancing thoroughness with practicality. Excessive documentation creates burden without adding value, while insufficient records leave gaps in your defense when challenged. Focus on capturing information actually used for decision-making and verification.
How GMP Shows Up on the Production Floor
Understanding GMP in theory means nothing if you can't recognize it in daily operations. Let's walk through what strong GMP looks like in action.
Watch your receiving area during a raw material delivery. You'll see staff checking temperatures, inspecting packaging integrity, verifying supplier certifications, and documenting findings before accepting loads. They're not just moving materials—they're executing your first critical control preventing contaminated ingredients from entering production.
On the production floor, you'll notice color-coded tools preventing cross-contamination between areas, cleaning schedules posted and checked off, temperature monitoring devices connected to critical equipment, and hand-washing stations positioned at strategic locations. Equipment gleams from recent sanitation, and maintenance tags show current calibration status.
Daily GMP execution you should observe includes line checks at startup verifying equipment cleanliness, in-process monitoring with results recorded in real time using Allera's digital Forms, allergen changeover procedures with thorough cleaning and verification testing, metal detector testing at scheduled intervals with documented results, and environmental monitoring swabs collected from non-product surfaces.
Your supervisors conduct GMP observations, providing immediate feedback when they spot deviations. Quality staff perform routine audits checking documentation completeness. Leadership makes regular floor visits asking questions that reinforce GMP importance rather than just touring with their hands behind their backs.
How FSMA, FDA, and GFSI Standards Enforce GMP
You're navigating multiple regulatory and certification frameworks, all reinforcing GMP expectations. Understanding how these systems interconnect helps you build efficient programs satisfying multiple requirements simultaneously.
FDA's Role in GMP Enforcement
The FDA establishes baseline GMP requirements for food facilities through regulations in 21 CFR Part 117. These regulations carry legal force—violating them can result in warning letters, consent decrees, product seizures, and even criminal prosecution.
FDA enforcement tools affecting your operations include routine inspections of registered food facilities, for-cause inspections triggered by complaints or illness outbreaks, warning letters publicly documenting violations, import alerts blocking products from entering U.S. commerce, and mandatory recalls when voluntary actions are inadequate.
When FDA investigators visit your facility, they're evaluating GMP compliance through direct observation, document review, and staff interviews. They're not just checking whether you have programs on paper—they're verifying actual implementation. You can't fake GMP compliance during FDA inspections. Investigators are trained to spot inconsistencies between written procedures and actual practices.
FSMA's Impact on GMP Compliance
The Food Safety Modernization Act shifted FDA's focus from responding to contamination events toward preventing them. This preventive approach elevated GMP's importance as foundational to effective food safety management.
FSMA's Preventive Controls Rule explicitly requires that you establish and implement GMP before developing your food safety plan. You can't identify hazards and implement controls if your basic manufacturing environment isn't sanitary and controlled.
FSMA connections to your GMP program include environmental monitoring requirements depending on effective sanitation GMP, allergen controls building on personnel hygiene and equipment cleaning GMP, supply chain verification assuming suppliers maintain adequate GMP, and recall effectiveness relying on traceability records as part of GMP documentation.
GFSI Standards and GMP Alignment
Global Food Safety Initiative benchmarked schemes like SQF, BRC, and FSSC 22000 have become de facto requirements for supplying major retailers. These certification standards incorporate GMP requirements often exceeding basic regulatory minimums.
GFSI schemes elevate GMP expectations through detailed prerequisites covering facility design and maintenance, specific requirements for pest management program elements, mandatory environmental monitoring in ready-to-eat production environments, staff hygiene standards including health screening and reporting, and cleaning validation requirements proving sanitation effectiveness.
Your GFSI auditor scrutinizes GMP implementation as foundation for your entire food safety management system. Weak GMP creates major or critical nonconformances that can cost you certification or require intensive corrective action.
Common GMP Failures That Lead to Audit Findings
Let's get brutally honest about where you're most likely to fall short. Certain GMP failures appear repeatedly in audit findings across the food industry.
Personnel hygiene violations consistently top the list: employees wearing jewelry or watches despite written prohibitions, inconsistent handwashing with staff skipping sinks or using inadequate technique, personal items like phones in production zones creating contamination risks, and improper use of gloves including not changing between tasks.
Facility and equipment issues that repeatedly surface:
- Peeling paint or damaged floor tiles creating harboring areas
- Inadequate lighting making cleaning verification impossible
- Standing water from poor drainage or leaking equipment
- Equipment not accessible for thorough cleaning with residue building up
- Uncalibrated monitoring equipment providing unreliable data
Documentation gaps that undermine your defense include incomplete monitoring records with blanks or entire sections filled out obviously at shift end, missing batch records for produced products, calibration records showing overdue equipment, training records lacking detail about competency verification, and corrective action records showing problems identified but never closed with verification.
Process control failures create real food safety risks: time and temperature abuse with products held too long at unsafe temperatures, allergen cross-contact from inadequate changeover cleaning, rework added back without proper evaluation, and products released before testing results confirm safety.
Here's what's insidious about these failures: individually, they might seem minor. But auditors don't evaluate issues in isolation. They're looking for patterns revealing inadequate systems. Multiple minor findings in the same category demonstrate that your programs aren't effectively implemented, regardless of what your procedures claim.
How to Assess GMP Gaps in Your Facility
Before you can fix GMP problems, you need accurately diagnosing where gaps exist. Many facilities operate with blind spots—areas where leadership believes programs are solid but reality tells a different story.
Start with a comprehensive baseline assessment. Conduct a thorough GMP audit covering all program elements. Don't delegate this entirely to your quality team. You need fresh eyes, either from corporate resources, consultants, or industry peers willing to exchange facility assessments. Internal teams often overlook problems they see daily, having normalized conditions that would shock external auditors.
Assessment methods uncovering hidden problems include physical facility walkthroughs during production observing actual practices rather than sanitized demonstrations, employee interviews away from supervisors revealing real behaviors and understanding levels, document review examining completeness and consistency of records, process observations watching complete production sequences, and mock recalls testing whether your traceability actually works under pressure.
Pay special attention to production practices during night shifts, weekends, and peak demand periods. GMP often deteriorates when regular management isn't present or when pressure to meet schedules intensifies.
Critical questions your assessment must answer: Are written procedures actually followed, or do unwritten "real" procedures exist? Do employees understand why GMP matters, or do they view it as bureaucratic hassle? Are deviations promptly identified and corrected, or do they persist because nobody addresses root causes?
Once you've identified gaps, resist the temptation to attack everything simultaneously. You need prioritizing based on food safety risk, regulatory exposure, and feasibility of correction. Create a gap closure plan with specific corrective actions, assigned responsibilities, target completion dates, and verification methods.
How to Implement and Roll Out GMP
Whether you're establishing GMP programs from scratch or overhauling inadequate systems, successful implementation requires careful planning and execution.
Building Your GMP Framework
Start by defining your program scope and structure. What specific GMP elements do you need based on your products, processes, and regulatory requirements? Don't copy generic templates without adapting them to your actual operations.
Framework development steps include mapping your current processes identifying all points where GMP controls are needed, researching applicable requirements from FDA regulations and GFSI schemes, benchmarking against industry best practices learning from others' successes, drafting comprehensive written programs covering all GMP categories, and designing supporting forms and records enabling consistent execution.
Your written programs should be practical and usable, not theoretical documents gathering dust. Write procedures at the comprehension level of the employees who'll execute them. Include photos, diagrams, and visual aids making expectations crystal clear.
Training and Culture Development
Even brilliant programs fail without effective training building both competence and commitment. You need employees who can correctly perform GMP activities and genuinely believe in their importance.
Your multi-layered training approach should include initial onboarding covering facility-specific GMP requirements before new employees enter production, role-specific training going deeper into practices relevant to each job function, regular refresher training reinforcing critical practices and addressing performance gaps, just-in-time training when procedures change, and hands-on demonstration and practice, not just classroom lectures.
Beyond formal training, you're shaping food safety culture through daily actions. When supervisors conduct GMP observations and provide immediate feedback, they're reinforcing standards. When leadership responds seriously to GMP concerns, they're demonstrating importance.
Phased Implementation Approach
Rolling out comprehensive GMP simultaneously across your entire facility risks overwhelming your organization. Consider phased implementation allowing you to build momentum and learn from early efforts.
Potential phasing strategies include starting with highest-risk areas like ready-to-eat production before addressing lower-risk zones, implementing category-by-category, perhaps beginning with personnel hygiene before tackling complex process controls, or deploying to one production line as a pilot, refining approaches before expanding facility-wide.
Whatever sequence you choose, maintain realistic timelines. GMP implementation requires not just writing procedures but changing behaviors, upgrading facilities, and building systems. Communicate progress transparently, share implementation milestones, celebrate successes, and honestly address challenges.
How to Improve GMP with Audits and CAPA
Implementing GMP is just the beginning. You need continuous improvement systems ensuring your programs remain effective as conditions change.
Schedule regular internal GMP audits covering all program elements on a defined frequency. These aren't "gotcha" exercises designed to catch people failing—they're systematic assessments identifying opportunities for strengthening your systems. Effective internal audits combine announced evaluations examining documentation with unannounced observations capturing actual daily practices.
Your auditors should be trained and qualified, understanding not just what to look for but how to assess root causes behind findings. When they discover an incomplete monitoring record, they're not just documenting the missing entry—they're investigating why it wasn't completed and whether systemic issues exist.
Every identified GMP gap should trigger your CAPA (Corrective and Preventive Action) process, and the fastest way to keep CAPA traceable is to link corrective actions back to the original Digital Form submission and the related controlled SOP in document control. This structured approach ensures problems receive thorough investigation, effective correction, and verification preventing recurrence.
Robust CAPA addresses:
- Immediate correction fixing the specific problem instance
- Root cause analysis determining why the problem occurred
- Corrective action eliminating the root cause
- Preventive action preventing similar problems in related areas
- Verification confirming actions were implemented and actually effective
Your CAPA system should track findings from multiple sources: internal audits, external audits, regulatory inspections, customer complaints, and employee reports. This comprehensive view reveals patterns that might not be obvious looking at single incidents.
Why Paper-Based GMP Programs Break Down
Many facilities still rely on paper forms, clipboards, and filing cabinets for GMP documentation. If you're among them, you're fighting uphill battles that undermine program effectiveness.
Paper-based systems create predictable problems. Records get easily lost or damaged, leaving gaps in your compliance defense. Illegible handwriting makes verification impossible. Staff fill forms at shift end rather than real-time, defeating the purpose of monitoring. You have no trend analysis capabilities without tedious manual data entry. Retrieving records during audits or investigations becomes frantic searches through filing cabinets. There are no alerts when monitoring isn't performed or limits are exceeded. Accountability for incomplete documentation is virtually non-existent.
Perhaps most critically, paper systems make it easy to hide problems until they become crises. When deviations occur, they're often addressed locally without triggering broader investigation. You lose opportunities for learning and preventing recurrence across your facility.
Paper also creates administrative burden diverting resources from value-added activities. Your quality staff spends hours reviewing and filing forms rather than analyzing trends and driving improvement. Is that really the best use of their expertise?
Why Digital GMP Beats Paper and Spreadsheets
Digital GMP solutions transform how you manage food safety programs, creating capabilities impossible with paper or even spreadsheets.
Digital advantages you'll immediately notice include real-time data capture ensuring accuracy and completeness, automated alerts when monitoring isn't completed or limits are exceeded, instant access to records from anywhere for audits or investigations, trend analysis revealing patterns before they become problems, and electronic workflows routing issues to appropriate personnel automatically.
Beyond operational efficiency, digital systems enable proactive management. You're not discovering problems when auditors point them out—you're identifying and correcting them before external stakeholders notice. Digital platforms support better decision-making through data visualization. Instead of flipping through binders looking for patterns, you're viewing dashboards highlighting where attention is needed.
The training advantages are substantial too. Digital systems can include embedded job aids, videos, and reference materials employees access exactly when needed. You can track not just who completed training but also comprehension through integrated assessments.
Perhaps most importantly, digital GMP creates transparency that drives accountability. When everyone knows their performance is visible and tracked, behaviors improve. Leaders can recognize excellence and address deficiencies with objective data rather than subjective impressions.
Which GMP Metrics Food Manufacturers Should Track
You can't improve what you don't measure. Effective GMP management requires tracking metrics revealing program health and improvement opportunities.
Leading indicators show prevention effectiveness: GMP observation completion rates revealing whether monitoring is actually performed, training completion rates ensuring all personnel maintain current qualifications, preventive maintenance completion rates preventing equipment failures, environmental monitoring results detecting sanitation effectiveness before product contamination, and internal audit scores identifying gaps before external auditors find them.
Lagging indicators reveal outcomes that have already occurred. These include regulatory inspection findings and warning letters, customer complaints related to quality or contamination, product recalls and withdrawals, failed finished product testing requiring investigation, and external audit nonconformances from GFSI or customer assessments.
Don't just track metrics—analyze trends over time and across different areas of your facility. Where are you improving? Where are you backsliding? Which areas consistently outperform others, and what can you learn from them that's transferable?
Set targets for critical metrics and hold leadership accountable for achieving them. GMP performance should factor into performance evaluations and compensation decisions, demonstrating organizational commitment beyond words.
How Allera Supports Digital GMP in Food Manufacturing
Allera helps food manufacturers move GMP programs from paper-based compliance to streamlined, digital operations that are easier to manage, audit, and improve.
Centralized GMP Management
Allera brings your entire GMP program into one cloud-based platform, accessible from any device. Teams can complete GMP checks directly on the production floor using mobile devices—eliminating clipboards, duplicate data entry, and after-the-fact form completion.
Digital GMP Forms and Checklists
Allera provides configurable GMP templates and digital checklists based on food industry best practices. These can be customized to match your facility layout, products, and risk profile while enforcing consistent execution across shifts and lines.
Automated Workflows and CAPA
When issues are identified, Allera automatically routes them to the right personnel for review and corrective action. CAPAs are tracked from identification through closure, with time-stamped records and evidence attached for audit readiness.
Real-Time Visibility and Dashboards
GMP data is captured in real time and displayed through dashboards that show compliance status, trends, and recurring issues. This gives teams immediate visibility into where problems exist and whether corrective actions are working.
Training and Competency Tracking
Allera supports training and competency documentation by linking GMP tasks to personnel qualifications. This helps ensure the right people are performing GMP activities and that training records are readily available for audits.
Environmental Monitoring Support
Environmental monitoring activities can be managed through digital data capture and tracking workflows. Results are stored centrally, making it easier to review historical data and identify emerging trends that may require attention.
Audit and Inspection Readiness
Allera simplifies internal audits and external inspection preparation by centralizing records, corrective actions, and supporting evidence in one system. Gap assessments, follow-ups, and closures are easy to document and demonstrate.
Turning GMP Data Into Action
Instead of static paperwork, Allera transforms GMP data into actionable insights. Teams can spot risks earlier, focus resources where they matter most, and continuously improve GMP performance over time.
Conclusion
GMP is not just a regulatory requirement. It is the baseline for running a controlled, reliable food manufacturing operation. As expectations increase in 2026, the facilities that perform best will be the ones that treat GMP as part of daily operations, not something managed only for audits.
GMP touches everything on the floor: how people work, how equipment is maintained, how processes are controlled, and how problems are documented and fixed. Regulations like FSMA and GFSI make these expectations explicit, but many failures still come from the same place—limited visibility and slow, manual systems.
Paper-based GMP makes it difficult to see issues early, follow up consistently, or prove that controls are working. Digital GMP changes that by giving teams timely data, clear ownership, and traceable actions. Instead of reacting after something goes wrong, facilities can manage risks as they develop.
At its core, GMP is about consistency and accountability. When systems make it easier to do the right thing every day, compliance follows naturally. Whether you are improving an existing program or starting fresh, strong GMP comes from building controls into operations and using tools that support—not slow down—your team.
The real risk isn’t investing in GMP.
It’s operating without enough control to know when something is going wrong.
FAQs
What are the 5 principles of GMP?
The 5 principles of Good Manufacturing Practices (GMP) focus on building consistent, safe, and controlled manufacturing operations. They are:
- People – Employees must be properly trained, qualified, and follow hygiene and safety practices to prevent contamination and errors.
- Processes – Manufacturing processes must be clearly defined, validated, and consistently followed to ensure repeatable results.
- Premises & Equipment – Facilities and equipment must be designed, maintained, and cleaned to prevent contamination and support safe production.
- Products & Materials – Raw materials, ingredients, packaging, and finished products must meet defined specifications and be properly controlled.
- Procedures & Documentation – Written procedures, records, and controls must exist to prove work was performed correctly and consistently.
Together, these principles ensure food is produced safely, legally, and to quality standards.
What are the FDA GMP requirements?
FDA GMP requirements are legally enforceable standards that food manufacturers must follow under 21 CFR Part 117 (FSMA Current Good Manufacturing Practices). Key requirements include:
- Personnel hygiene and training
- Sanitary facility design and maintenance
- Equipment suitability, cleaning, and maintenance
- Process controls to prevent contamination
- Allergen control programs
- Sanitation and environmental monitoring
- Pest control
- Supply-chain controls
- Recordkeeping and documentation
- Corrective actions for deviations
Failure to meet FDA GMP requirements can result in warning letters, product seizures, recalls, or facility shutdowns. GMP compliance is the foundation of FDA food safety enforcement and is required regardless of company size.
What are the 4 main components of GMP?
The 4 main components of GMP are the core building blocks regulators expect to see in any compliant facility:
- Personnel – Training, hygiene, supervision, and accountability of employees.
- Facilities & Equipment – Proper design, maintenance, sanitation, and calibration to prevent contamination.
- Processes & Controls – Defined production steps, monitoring, preventive controls, and corrective actions.
- Documentation & Records – SOPs, logs, batch records, and verification documents that demonstrate compliance.
If one of these components is weak, GMP failures and audit findings are likely.
What are the 10 rules of GMP?
The 10 commonly accepted rules of GMP provide practical guidance for daily operations:
- Write clear procedures and follow them.
- Keep accurate, complete, and timely records.
- Maintain clean and sanitary facilities.
- Use equipment that is suitable, maintained, and calibrated.
- Train employees and document competency.
- Control raw materials and suppliers.
- Prevent cross-contamination and mix-ups.
- Investigate deviations and implement corrective actions.
- Verify processes through monitoring and audits.
- Practice continuous improvement, not one-time compliance.
These rules turn GMP from theory into operational discipline.

.avif)










%20Checklist%20(2).avif)

.avif)


.avif)