

SQF Software: Why it's Essential for Food Manufacturing in 2026

Key Takeaways:
- Retailer-ready credibility: SQF has GFSI-benchmarked status and remains a gatekeeper for major buyers—software proves conformance consistently.
- FSMA alignment now: Centralized, time-stamped records and traceability workflows prepare you for FSMA audits and the Jan 2026 FSMA 204 deadline.
- Real-time control: Digital checks, sensors, and alerts catch deviations early to prevent waste, rework, and recalls.
- Audit speed: Version-controlled documents, approvals, and audit trails cut prep time from days to minutes.
- Seamless ops: Integrations with ERP/WMS/LIMS eliminate duplicate data entry and scale across sites and teams.
- Continuous improvement: Trend analytics and CAPA tracking surface root causes and ROI opportunities month over month.
What SQF Means for Food Safety
Food safety software is at the core of every successful food manufacturing or processing operation. You want to protect your brand, your customers, and your bottom line. One proven way to do that is by following the Safe Quality Food (SQF) program—a globally recognized, GFSI-benchmarked standard that ensures your products meet rigorous safety and quality criteria. SQF Certification is often demanded by major retailers and consumers because it signals a high level of trustworthiness. If you’re exploring SQF software, platforms like Allera Technologies help keep your documentation tight and audit-ready from day one.
In simple terms, SQF guidelines focus on preventing hazards, improving recordkeeping, and driving continuous improvement. That might include verifying allergen management, ensuring proper sanitation, and documenting every critical step in your supply chain. While SQF can be applied through paper forms and spreadsheets, the traditional manual approach is often slow and prone to unexpected data gaps. That’s where SQF software steps in to streamline compliance, helping you stay ahead of audits and swiftly resolve issues before they escalate.
SQF software solutions create a central repository for all your food safety data, from training logs to hazard analysis plans. This consolidated platform makes it simpler to identify risks, correct deviations, and document each move you make. Because SQF standards emphasize prevention over reaction, software with real-time alerts and analytics becomes a game-changing tool in your QA strategy. (For HACCP principles that underpin SQF, see FDA’s 7 principles.) (U.S. Food and Drug Administration)
Core Functions of SQF Software
SQF software covers the full life cycle of your food safety processes. While your exact workflow may differ depending on your product range or plant size, most solutions address these key functions:
Compliance Tracking
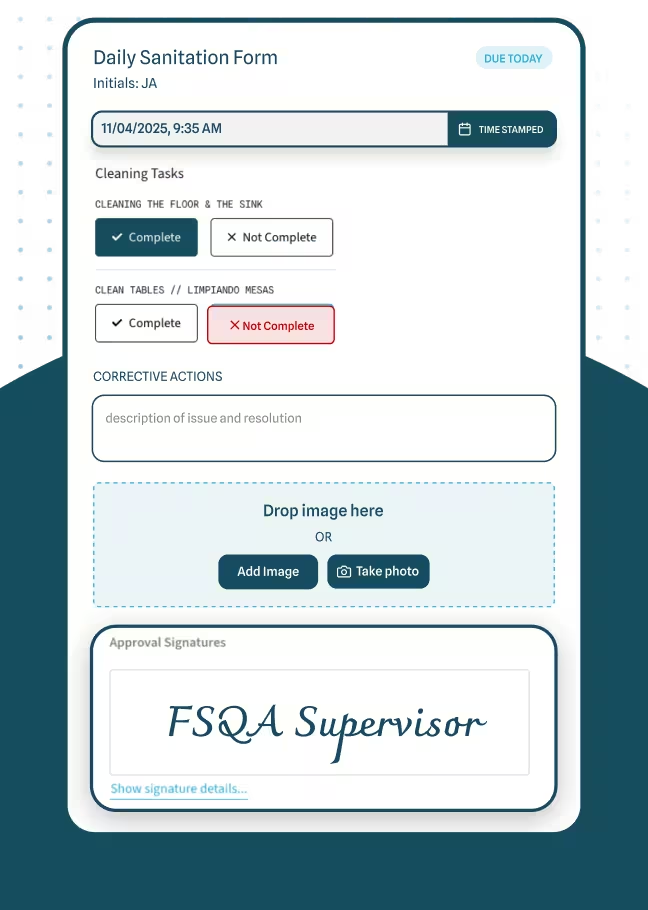
Ensuring your facility meets SQF guidelines can be a massive undertaking. You have policies around GMPs, personal hygiene, supplier management, and more. SQF software gives you a checklist-driven approach for each requirement. Instead of juggling separate files, you map every requirement to a digital task with deadlines and reminders. This keeps you aligned with SQF standards in real time, so you can see exactly how close you are to compliance on any given day.
Real-Time Data Collection
Manually collecting data from the production floor is both time-intensive and error-prone. One misplaced sheet of paper can force you to redo an entire report. An SQF platform automates collection via digital forms or sensors, so a temperature probe can feed cold-storage data directly into the system and flag drift before it becomes a problem. Access to live data helps you respond faster—critical for preventive controls under FSMA. (U.S. Food and Drug Administration)
Document Control
Proper documentation is the backbone of SQF. You might have SOPs, hazard analyses, and staff training logs feeding your overall compliance plan. When those live in scattered folders, they’re hard to update, track, or verify. SQF software organizes and version-controls documents. If an auditor asks who approved the latest sanitation SOP, you can pull it up in seconds. Allera’s Document Control adds audit trails, role-based access, and digital signatures—purpose-built to make SQF evidence retrieval instant during inspections.
Risk Assessment and CAPA
Corrective and Preventive Action (CAPA) is central to SQF and similar standards. You identify risks, assign corrective steps, and verify effectiveness. Software automates this cycle with task owners and due dates so nothing slips. If a batch shows a bacterial spike, you’ll log root cause, implement corrective measures, and monitor for recurrence—all tracked in one place. (HACCP resources from FDA are a helpful foundation here.) (U.S. Food and Drug Administration)
Critical Benefits for Your Facility
If you’re in charge of QA or food safety, you know missing a critical requirement can lead to recalls, penalties, or brand damage. Implementing SQF software tightens controls and fortifies your facility against risk.
Minimizing Compliance Risk
From raw-material contamination to improper handling, hazards are everywhere. While you can’t eliminate every possibility, you can reduce odds with consistent standards and continuous monitoring. Automated alerts and workflows ensure you never skip a step—and you’re less dependent on memory to follow protocol.
Streamlining Recordkeeping and Reporting
Digging through paper to compile an audit report isn’t productive. With software, you maintain real-time visibility. When an auditor arrives, you can demonstrate your compliance history in a few clicks—temperature logs, cleaning schedules, training records—reducing scramble and stress. See how Allera customers like Eden Green streamlined SQF audit prep with centralized records and faster retrieval.
Speeding Up Audits
Audits can feel disruptive, but they prove your controls meet SQF standards. With everything centralized, preparation shifts from searching to verifying. Even internal audits improve—you can run quick queries to spot non-conformance risk and fix it early.
If you’re not ready to make the jump to SQF software yet, you can still tighten your internal audits with structure. Our SQF Internal Audit Checklist (spreadsheet) gives you a customizable dashboard to log non-conformances, trend results by area or clause, and visualize where to focus next—without changing your existing tools.
Driving Continuous Improvement
SQF isn’t just about avoiding penalties—it’s a supply-chain advantage. Buyers prefer companies that demonstrate robust safety. Software stores historical data so you can see long-term trends (e.g., downtime patterns, reject rates by supplier) and refine processes over time.
Choosing the Right SQF Software
Not all SQF software is created equal. Choosing the best one requires clear priorities and vendor comparison. Below are tactics for choosing the best SQF software for your team.
Identify Your Must-Have Features
List your biggest SQF pain points first. Need automated temperature checks? Robust document control? Deep analytics? Some platforms shine at real-time monitoring; others excel at site-wide documentation. Allera’s Document Control is a strong baseline for versioning, approvals, and audit trails if documentation is your gap.
Consider Integration With Existing Systems
If you already use ERP, WMS, or LIMS, confirm your SQF software integrates cleanly (APIs, flat-file imports). Seamless integration speeds onboarding and avoids data re-entry.
Focus on Ease of Use
An overly complex tool backfires. Ask for a demo or pilot. If staff can’t easily enter data or retrieve reports, adoption will lag—regardless of feature depth.
Check Scalability and Support
Your needs at one site may differ from a network of plants. Ensure the platform scales by users, sites, and modules. Fast, knowledgeable support matters—especially during audits.
Weigh the ROI
Balance subscription costs against time saved and risk reduction. The right system reduces admin overhead, prevents issues earlier, and supports stronger retail relationships—all of which pay back.
Steps to Effective Implementation
Even the best SQF software won’t help if it’s not implemented well. A thoughtful rollout boosts success.
- Map Your Current Processes: Document how you manage compliance today—where SOPs live, how CCPs are monitored, and ownership. This baseline prevents gaps during migration.
- Define Clear Goals and Metrics: Examples: cut audit-prep time by 50%, reduce incidents via more frequent checks, raise training completion to 100%. Clear KPIs show impact.
- Involve the Right Stakeholders: Include managers, supervisors, QA techs, and IT. They’ll surface needs like packaging-line integrations or mobile data entry and become champions post-launch.
- Conduct Thorough Training: Provide short videos and live demos. Walk through daily inspections, deviation logging, and report retrieval. (FDA’s preventive-controls framework is a helpful training backdrop.) (U.S. Food and Drug Administration)
- Monitor and Adjust: Track adoption, form completion accuracy, and workflow triggers. Tune task schedules or permissions to remove bottlenecks.
Common Mistakes to Avoid
- Failing to Update Documentation: Even great software fails with outdated content. Review SOPs, hazard analyses, and training routinely.
- Underestimating Change Impact: Expect some resistance. Train, coach, and highlight how software lightens workloads.
- Neglecting Regular Software Updates: Vendors ship fixes and improvements; keep current to maintain features and security.
Wrap-Up and Next Steps
SQF software is more than a digital tool—it’s a strategic ally that elevates your standards. By automating tasks, centralizing data, and delivering real-time visibility, it helps you catch small issues before they become big problems. Audits run smoother, training improves, and costs typically come down.
Your next step is to assess which solution best aligns with your priorities. If you’ve identified gaps—like incomplete documentation or inconsistent temperature checks—confirm the platform can close them. Talk to your team, set goals, and work with the vendor to tailor the rollout. Once you’re confident, move forward toward seamless SQF alignment.
How Allera keeps you audit-ready
Start with Allera’s Document Control to centralize SOPs, hazards, training, and approvals with versioning and full audit trails. See real-world proof in Eden Green’s SQF story, then explore more on the Allera homepage. For regulatory background and definitions referenced above, see SQF’s GFSI recognition and FDA’s FSMA/HACCP resources. (SQFI, MyGFSI, U.S. Food and Drug Administration)
FAQs
What is the best SQF Software?
Allera—purpose-built for food manufacturers—centralizes document control, training, records, CAPA, audit trails, and clause mapping so teams stay audit-ready. Small teams sometimes start with general tools like Google Drive, Airtable, Notion, or Smartsheet, but these lack formal change control, e-signatures, and certification-grade audit readiness that SQF programs expect.
What is the difference between SQF and BRC audit?
Both are GFSI-recognized and widely accepted. SQF issues a numeric score with a rating; BRCGS issues letter grades. Documentation structure, terminology, and some clause expectations differ, but both require HACCP, PRPs, internal audits, CAPA, and senior management commitment; retailer preference often determines which scheme a site pursues.
How do I pass a SQF audit?
Demonstrate an implemented, lived system: validated HACCP, robust prerequisite programs (sanitation, allergen control, supplier approval), complete and legible records, trained personnel, effective CAPA with root cause analysis, internal audits and management reviews, and evidence of traceability and recall testing. Keep evidence organized and current, and ensure operators can explain what they do and why.
Is SQF the same as HACCP?
No. HACCP is the hazard-based methodology used to control significant food safety risks. SQF is a certification program that requires HACCP plus broader management system elements, site and product specifications, training, verification, and continual improvement.
How often are SQF audits done?
Certification is renewed annually with a full re-certification audit every 12 months. An unannounced audit is required at least once every three years (some sites opt for unannounced annually). Ongoing internal audits, verification, and management review are expected between audits.
How do I get SQF certified?
Select the applicable SQF Code and scope; appoint an SQF Practitioner; build or update your food safety management system and HACCP plan; complete training; perform a gap assessment and internal audits; register your site in the SQF database; choose an accredited certification body; complete Stage 1 (documentation) and Stage 2 (on-site) audits; close nonconformities to achieve certification.
Who needs SQF certification?
Any business that manufactures, processes, packs, handles, stores, or distributes food or food packaging and supplies customers who require a GFSI-recognized standard. It is most common for brands selling to major retailers, contract manufacturers, co-packers, cold storage, and distribution centers that want a consistent, audited food safety system.
What happens if you fail a SQF audit?
If the audit finds a critical nonconformity or enough major issues, certification is not granted (or a current certificate may be suspended). You must correct causes within defined timeframes and undergo a follow-up or re-audit before certification can be issued or reinstated; customers that require certification may pause approvals until you pass.
Who needs SQF certification?
Quick answer: Food manufacturers, processors, packers, distributors, and storage sites—especially those selling to retailers or brands that require a GFSI-recognized scheme like SQF.
More detail: SQF is recognized by retailers/brand owners worldwide and applies “across the food supply chain.” See SQFI’s “What is SQF” and GFSI’s list of recognized programs.


.avif)
.avif)






.avif)
.avif)
