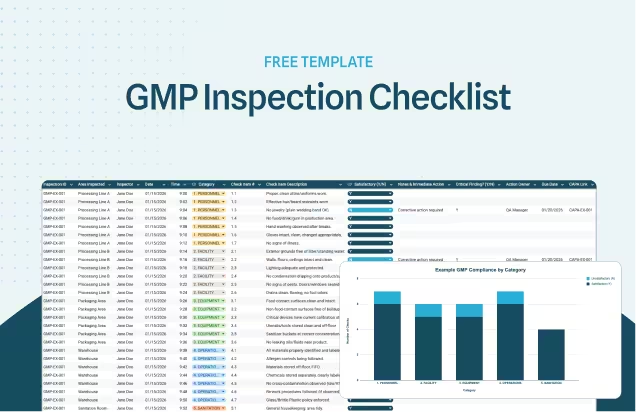
Guide to Quality Assurance in the Food Industry

Why is Food Quality Assurance Changing?
Let's be honest—quality assurance in food the food industry is changing faster than most of us ever anticipated.
Those three-ring binders stacked in your QA office? The annual supplier audits you've relied on for years? The paper checklists your team fills out every shift? They're all becoming relics of a system that simply can't keep up with today's regulatory expectations.
Here's what's driving this shift: New global standards, evolving FDA requirements, and technology that actually works have all converged at once. The result? Real-time verification isn't just a nice-to-have anymore—it's what auditors expect when they walk through your door.
This affects everyone in your operation:
- You're facing new documentation requirements
- Your suppliers need to meet higher transparency standards
- Your entire facility needs systems that can prove compliance instantly, not after someone spends three hours digging through filing cabinets
So what does quality assurance actually look like in 2026?
From Allera's experience working with over 500 food manufacturers and quality assurance teams, we've identified five major changes that are reshaping how food manufacturers approach compliance, documentation, and supplier management.
More importantly, we'll show you practical steps you can take right now to get ahead of these shifts instead of scrambling to catch up.
If you’re still defining what quality assurance actually means in practice, see our article on Food Quality Control vs. Quality Assurance.
What is Quality Assurance in the Food Industry?
Quality assurance in the food industry encompasses the systematic processes and procedures that ensure food products consistently meet safety standards, regulatory requirements, and customer expectations throughout the entire food and beverage supply chain—from raw material sourcing to final product delivery.
Unlike quality control, which focuses on identifying defects in finished products, quality assurance is preventive. It establishes the framework that prevents problems before they occur. This includes everything from supplier qualification and ingredient testing to sanitation protocols, employee training programs, and documentation systems that prove compliance.
The scope of food industry quality assurance has expanded dramatically in recent years. Modern QA programs must address:
Regulatory Compliance
Meeting FDA regulations, USDA requirements, and international standards that govern food safety and labeling across different markets and product categories.
Certification Standards
Maintaining certifications like SQF (Safe Quality Food), BRCGS (Brand Reputation through Compliance Global Standards), FSSC 22000, and customer-specific audit requirements that have become prerequisites for market access.
Risk Management
Identifying and mitigating biological, chemical, and physical hazards through HACCP (Hazard Analysis Critical Control Points) plans and comprehensive food safety programs.
Supply Chain Integrity
Verifying that every ingredient, package, and material entering your facility meets specifications and comes from approved, compliant sources.
Traceability Requirements
Maintaining the ability to track products forward to customers and backward to suppliers—a capability that's become non-negotiable under regulations like FSMA Section 204.
The stakes couldn't be higher. Quality failures in the food industry don't just result in audit findings or regulatory citations. They can trigger costly recalls, cause foodborne illness outbreaks, destroy brand reputation built over decades, and in severe cases, lead to criminal prosecution of executives and facility closures.
Change 1: Continuous Supplier Verification Replaces Annual Audits
Why Real-Time Supplier Compliance?
Remember the old routine? Schedule your annual supplier audit, review their documents once a year, file everything away, and forget about it until next year's audit cycle rolls around.
That approach is dying fast.
Regulatory bodies and certification schemes have figured out something important: Quality issues don't wait for convenient annual review dates.
- A supplier's food safety certificate can expire in June
- Their performance might deteriorate in September—which is why continuous oversight is recommended in the SQF Certification Body Directory.
- Critical certifications can lapse without anyone noticing—until it becomes your problem during an audit
Here's the shift: FSMA regulations and updated SQF standards now expect you to demonstrate ongoing supplier oversight.
When an auditor asks about your supplier's compliance status, they want to know what's happening right now—not what you verified six months ago.
What Continuous Supplier Verification Looks Like in Practice
Modern supplier verification operates in real-time, and it's honestly less stressful than the old way once you set it up.
Digital platforms automatically track supplier certifications, send alerts when documents are about to expire, and maintain a current compliance status for every vendor in your supply chain. Instead of manually chasing suppliers for updated certificates (we know that's nobody's favorite task), the system creates automated workflows that do the heavy lifting.
Here's what you see:
- Suppliers get notifications when their documents need renewal
- You see a dashboard showing which suppliers are current, which need attention, and which ones are creating potential compliance gaps
- No more spreadsheets, no more sticky notes, no more hoping you didn't miss something important
Performance scorecards add another layer that actually helps you do your job better. By tracking metrics like on-time document submission, nonconformity rates, and how quickly suppliers respond to corrective actions, you build a data-driven picture of each supplier's reliability. You'll quickly identify your rock-solid partners and the ones who might need additional support—or evaluation.
Platforms with supplier management centralize this entire process, automating expiration tracking and creating compliance reminders that keep your supplier data current year-round. When audit day arrives, you're not scrambling to collect documentation. You already have it, organized and ready to go.
Actionable Steps for Implementing Continuous Verification
Ready to modernize how you handle suppliers? Here's where to start:
Digitize your supplier onboarding process.
Create electronic submission requirements for all certifications, licenses, and quality documentation using a supplier management system. This builds a digital trail from day one and eliminates the chaos of tracking paper certificates across multiple suppliers.
Set up automated expiration tracking. Your system should flag approaching expiration dates at least 60-90 days in advance. This gives both you and your suppliers plenty of time to address renewals without the last-minute panic.
Establish clear performance metrics. Define what "good" supplier performance looks like at your facility, then track it consistently. Common metrics include:
- Document submission timeliness
- Quality incident rates
- How responsive they are to corrective actions
Build regular touchpoints into supplier relationships. Continuous verification doesn't mean you stop talking to your suppliers. It means your conversations are informed by real-time data, making them more targeted and productive. You're having strategic discussions, not just asking them where their paperwork is.
The Regulatory Landscape Driving Quality Assurance Evolution
Understanding why quality assurance in the food industry is changing requires context about the regulatory forces reshaping compliance expectations.
FSMA: The Paradigm Shift Toward Prevention
The FDA Food Safety Modernization Act (FSMA) fundamentally changed the regulatory approach from responding to contamination to preventing it. The law's key rules create specific QA obligations:
The Preventive Controls Rule requires manufacturers to implement written food safety plans identifying hazards and establishing controls to prevent them. Your quality assurance system must document these controls and prove they're working effectively.
The Foreign Supplier Verification Program (FSVP) places the burden of supplier compliance directly on importers. You can't simply trust that foreign suppliers meet U.S. standards—you must verify their compliance through your QA program.
The Intentional Adulteration Rule addresses food defense, requiring manufacturers to identify vulnerabilities where someone could deliberately contaminate products and implement mitigation strategies.
GFSI Recognition and Certification Schemes
The Global Food Safety Initiative (GFSI) benchmarks various certification schemes against recognized standards. Major retailers increasingly require suppliers to hold GFSI-recognized certifications, making these de facto mandatory for market access:
SQF emphasizes systematic approaches to food safety and quality management with rigorous documentation requirements.
BRCGS focuses on product safety, quality, and operational criteria with particular attention to traceability and supply chain management.
FSSC 22000 combines ISO 22000 requirements with sector-specific prerequisites for comprehensive food safety management.
These certification bodies conduct increasingly thorough audits that scrutinize not just whether you have procedures, but whether you can prove they're consistently followed and effective.
FDA Inspection Modernization
FDA food inspections themselves have evolved. The New Era of Smarter Food Safety initiative emphasizes technology-enabled traceability, predictive analytics, and real-time data sharing. Inspectors increasingly expect to see digital systems that provide immediate access to records rather than having to wait while someone retrieves files from storage.
Change 2: SOPs and Quality Assurance Training Must Be Digitally Linked
Why Traceability Between Training and Procedures Is Now Mandatory
Let's talk about a scenario that probably sounds familiar—and slightly terrifying.
An auditor asks to see proof that everyone working on Line 3 has been trained on the current version of your allergen control SOP. You pull training records showing that, yes, everyone completed allergen training last year. Then the auditor points out that your SOP was updated six months ago.
Can you prove everyone was retrained on the new version?
If you're relying on paper sign-off sheets and spreadsheet-based training logs, this question can quickly become a finding. And it's not hypothetical—regulators increasingly expect verifiable links between procedure updates and employee training.
Think about it this way:
When an SOP changes, there needs to be a clear, documented path showing how that change reached the affected employees and how you verified they understood it.
That's not easy to demonstrate when your training system and your document control system don't talk to each other.
How Digital Systems Create Seamless Training Documentation
Modern quality management platforms solve this by creating direct connections between your SOPs and training records.
Here's how it works: When you update a procedure, the system automatically identifies which employees need to be notified based on their roles and responsibilities—this is simplest when your Document Control and training workflows are connected
The workflow:
- It generates training assignments
- Sends notifications to the affected team members
- Tracks acknowledgments
- Every single step creates an audit trail
* Note: you control the automation here. It wont fire off generating crappy AI training assignments, it uses your updated SOPs and training records.
You can demonstrate not just that Sarah was trained on the allergen SOP, but specifically which version she was trained on and exactly when she acknowledged understanding the updates. That's the level of traceability auditors are looking for.
Version control becomes transparent and automatic. Each SOP exists as a living document with complete revision history. Training records link directly to specific versions, creating an unbreakable chain of evidence that satisfies even the most thorough auditor.
Allera links SOP updates directly to employee training workflows, automatically notifying the right people when procedures change and tracking acknowledgments at the individual level. This eliminates hours of manual work matching training dates to document versions while giving you exactly the traceability auditors demand.
Common Pitfalls to Avoid in Training Documentation
Even with digital systems, certain mistakes can undermine your training documentation. Let's talk about the big ones.
Don't treat all training equally. Not every SOP update requires full retraining. Minor clarifications might only need employee acknowledgment, while significant procedural changes demand hands-on training and competency verification. Your system should distinguish between these different levels.
Record more than just attendance. Showing that someone sat in a training session isn't enough. You need evidence they understood the material and can apply it correctly. This might include:
- Competency assessments
- Practical demonstrations
- Supervised performance periods
Don't overlook temporary workers. Temps, contractors, and seasonal employees often need the same training as permanent staff, but they sometimes fall through the cracks in documentation. Your digital system should track all workers regardless of employment status.
Change 3: Real-Time Quality Assurance Data Becomes the Food Industry Standard
Moving Beyond Paper-Based Quality Assurance Systems
Paper-based quality systems served the food industry well for decades. But let's be realistic—they're fundamentally incompatible with what auditors and regulators expect today.
Here's the test: When a quality issue comes up or an auditor asks to see records from a specific production lot, how quickly can you access that information?
With paper systems, the honest answer is usually "hours" or even "days." Someone needs to:
- Locate the right binder
- Find the correct date
- Copy relevant pages
- Compile everything into something usable
Meanwhile, production might be on hold, products might already be in distribution, and costs are piling up by the minute.
Digital QA systems change this completely. Every data point—from receiving inspections to finished product testing—is captured electronically with automatic timestamps, user identification, and lot traceability. When you need information, you pull it up in seconds, not hours.
This instant accessibility transforms your role. Instead of spending your time as a reactive record-keeper, you become a proactive quality manager with real-time insight into every aspect of production.
Creating a Digital Quality Assurance Infrastructure
Transitioning from paper to digital doesn't have to be overwhelming. Start with strategy, not scale.
Identify your highest-value forms—the ones you reference most frequently or that carry the most compliance risk. Common starting points include:
- Receiving inspections
- Critical control point monitoring
- Sanitation verification
Convert these first and you'll see immediate benefits.
When you design your digital forms, don't overcomplicate things initially. The goal is to replicate what already works on paper while adding the advantages of digital capture: timestamps, required fields, photo documentation, and electronic signatures.
Build in quality controls that paper simply can't provide. Digital forms can:
- Require supervisory review before submission
- Automatically flag out-of-specification results
- Prevent incomplete records from being saved
These features reduce errors and ensure data integrity without adding extra steps to your team's workflow.
Here's where it gets really powerful: integration. When your QA data connects to inventory management, production scheduling, and supplier systems, you create complete food traceability across your entire operation. You can instantly pull all information related to a specific lot, supplier, or time period—something that's impossible with paper-based systems.
Quick Wins for Transitioning to Digital Food Quality Assurance
Want to see immediate improvements? Focus here:
Convert your daily production logs and Critical Control Point monitoring forms first. These generate the most entries and get referenced constantly during audits or investigations. Digitizing them creates instant time savings and dramatically improves data accessibility.
Implement photo documentation requirements. Digital platforms make it easy to attach photos to inspection records. This visual evidence is invaluable during audits and investigations, providing context that written descriptions alone can't capture.
Create supervisor approval workflows. Require digital sign-off from shift supervisors or QA managers before records are finalized. This adds accountability while creating a clear approval trail—and it catches errors before they become permanent records.
Set up automatic report generation. Configure your system to compile daily, weekly, or monthly QA summaries automatically. This eliminates manual report creation and ensures leadership always has current quality metrics without you having to drop everything to pull numbers together.
Essential Components of a Modern Food Quality Assurance Program
While technology is transforming implementation, the foundational elements of quality assurance in the food industry remain consistent. Here's what every comprehensive QA program must include:
Prerequisite Programs
These are the basic conditions and activities necessary to maintain a hygienic environment. They form the foundation upon which HACCP and other food safety systems are built:
Sanitation Standard Operating Procedures (SSOPs) that detail cleaning schedules, methods, chemical concentrations, and verification procedures for every area of your facility.
Pest control programs with documented monitoring, prevention measures, and corrective actions when evidence of pest activity appears.
Supplier approval and monitoring processes that ensure incoming materials meet specifications and come from verified sources.
Allergen management programs that prevent cross-contact and ensure accurate labeling—critical given that allergens cause the majority of food recalls.
HACCP Plans
Hazard Analysis and Critical Control Points (HACCP) is the systematic approach to identifying and controlling food safety hazards:
- Hazard analysis identifies biological, chemical, and physical hazards associated with your specific products and processes.
- Critical Control Points (CCPs) are the steps where you can apply controls to prevent, eliminate, or reduce hazards to acceptable levels.
- Critical limits define the boundaries that separate safe from unsafe at each CCP.
- Monitoring procedures verify that CCPs remain under control during production.
- Corrective actions specify what happens when critical limits are breached.
Your quality assurance system must document all of this and prove through records that it's happening consistently.
Testing and Verification Programs
Quality assurance extends beyond monitoring to proving that controls work:
- Environmental monitoring programs test surfaces, equipment, and facility areas for pathogens or indicators that sanitation is inadequate.
- Finished product testing verifies that final products meet microbiological, chemical, and physical specifications before release.
- Validation studies demonstrate that your process controls actually achieve the intended food safety outcomes.
- Third-party laboratory testing provides independent verification of product safety and quality parameters.
Document Control and Records Management
- Documentation proves your quality assurance program exists and functions effectively:
- Controlled documents ensure everyone works from current, approved procedures with proper version control and change management.
- Record retention systems maintain compliance documentation for timeframes specified by regulations and certification standards.
- Audit trails demonstrate who did what, when they did it, and under what version of the procedure—creating accountability and traceability.
Change 4: AI-Assisted Compliance Will Become More Common
How AI Is Transforming Pre-Audit Preparation
Let's talk about audit prep—specifically, the weeks many of us spend manually reviewing documents before certification audits.
You know the drill: painstakingly comparing your SOPs against regulatory requirements, searching for gaps that might lead to findings. It's time-consuming, somewhat subjective, and inevitably some issues slip through that auditors identify later anyway.
AI is fundamentally changing this process. Modern AI tools can analyze your entire quality management system against relevant regulatory frameworks—FSMA, SQF, BRCGS, or customer-specific requirements—in a fraction of the time human review requires.
And here's what's particularly useful: these systems don't just flag obvious gaps. They identify:
- Subtle inconsistencies
- Ambiguous language that might confuse auditors
- Missing elements that even experienced QA managers might overlook
You get more thorough compliance verification in significantly less time.
Practical Applications of AI in Food Quality Systems
AI-assisted compliance works on multiple levels, and understanding what it can actually do helps you use it effectively.
At the most basic level, these tools perform clause-by-clause analysis of your SOPs, checking whether your documented procedures address every required element of your certification scheme. That alone saves hours of manual cross-referencing.
More sophisticated applications identify risk areas based on industry trends and common audit findings. The AI might flag a procedure because similar language led to findings at other facilities—even if it technically meets requirements. This predictive capability helps you address potential issues before auditors raise them.
Document consistency checking is another valuable application. AI can identify:
- Contradictions between different SOPs
- Mismatches between procedures and forms
- Gaps where one document references requirements that aren't fully addressed elsewhere in your system
These are the kinds of issues that are surprisingly easy to miss when you're working with dozens of interconnected documents.
Allera's AI-assisted compliance feature automatically reviews SOP language against SQF clauses and other standards, flagging potential nonconformities before they become audit findings. This proactive approach helps you update documentation continuously rather than scrambling before certification audits.
Choosing the Right AI Tools for Your QA Team
Not all AI compliance tools are created equal, so here's what to look for:
Food industry specialization matters. General-purpose AI might miss nuances in food safety and quality assurance (FSQA) standards that specialized tools catch immediately. You want a system that understands the specific language and requirements of FSMA, SQF, and other food safety frameworks.
Transparency is crucial. The AI should explain why it flagged something, not just identify potential issues. You need to understand the reasoning so you can make informed decisions about what changes are actually necessary.
Integration capability is essential. AI compliance tools work best when they can access your actual documents and procedures, not just sample uploads. This allows for continuous monitoring rather than periodic manual reviews.
Keep the learning curve reasonable. The best AI tools augment your expertise rather than requiring you to become an AI specialist. Look for intuitive interfaces that provide actionable recommendations without overwhelming you with technical jargon.
Change 5: Predictive QA and Supplier Analytics Drive Prevention
From Reactive to Predictive Quality Management
Traditional quality assurance is inherently reactive. Something goes wrong, you investigate what happened, you fix it, and you move forward. This approach prevents the same problem from recurring, which is valuable—but it doesn't prevent new issues from emerging.
Predictive QA flips this model on its head.
By analyzing trends across your quality data, you can identify patterns that indicate emerging problems before they cause actual failures. It's the difference between fixing a breakdown and preventing one from happening in the first place.
Think about it this way: When you capture detailed information about every quality check, supplier delivery, and production lot over time, patterns start to emerge.
You might notice:
- Certain suppliers show gradual increases in noncompliance
- Specific production lines exhibit slow performance degradation
- Particular times of year correlate with quality issues
Armed with these insights, you can intervene proactively. Address supplier issues before they lead to rejected deliveries. Schedule equipment maintenance before performance drops enough to cause problems. Adjust staffing or training before seasonal challenges create quality incidents.
Key Metrics for Predictive QA in 2026
Building a predictive quality system starts with tracking the right metrics. Here's what actually matters:
Supplier noncompliance rates reveal patterns in vendor reliability. Track not just whether suppliers are compliant right now, but how often they've had expired documents, delayed submissions, or quality issues over the past six to twelve months. Increasing noncompliance suggests a supplier heading toward bigger problems.
CAPA closure time measures how quickly your team and your suppliers address issues. Long or increasing closure times indicate systemic problems with your corrective action process or supplier responsiveness. This metric helps you identify where you need additional resources or process improvements.
Audit finding rates over time show whether your quality system is strengthening or weakening. Track findings by category and you can focus improvement efforts on areas with persistent issues instead of treating every finding as an isolated incident.
Incident frequency and severity trends can reveal whether specific products, processes, or time periods present higher risks. This information guides where you allocate resources and where preventive action will have the most impact.
Allera's analytics tools let QA teams monitor these metrics continuously, spotting supplier performance issues, trending nonconformities, and systemic patterns that show exactly where preventive action will deliver the most value.
Building a Data-Driven Quality Culture
Here's the thing: metrics alone don't create predictive quality systems. You need a culture that values data, acts on insights, and continuously improves.
This starts with leadership commitment to using quality data for decision-making, not just compliance documentation. When executives ask "What do the numbers tell us?" instead of just "Are we compliant?", you know the culture is shifting.
Make data accessible. Quality metrics shouldn't be locked in the QA manager's office. Share relevant information with production teams, supervisors, and suppliers. When everyone understands performance trends, everyone can contribute to improvement.
Act on insights promptly. Predictive systems only deliver value if you respond to what they reveal. When data suggests an emerging issue, investigate and intervene quickly. This builds confidence in the system and prevents small problems from becoming major incidents.
Close the feedback loop. When predictive insights lead to interventions, track whether those actions achieved the intended results. This continuous improvement cycle makes your predictive models more accurate over time and demonstrates the concrete value of data-driven quality management.
The Technology Behind Modern Food QA Systems
What to Look for in a QA Management Platform
Choosing the right quality management platform is genuinely one of the most important decisions you'll make. The right system streamlines compliance, reduces manual work, and provides the traceability modern standards demand.
For an overview of leading options, explore our roundup of the 7 Best Food Quality Management Software for 2026
The wrong system? It creates frustration, duplicate data entry, and compliance gaps that make your job harder.
Here's what to prioritize:
Food industry specificity is non-negotiable. Generic quality management systems lack specialized features you actually need—things like:
- Allergen tracking
- HACCP plan management
- Alignment with food safety certification schemes
Don't settle for something that was designed for manufacturing widgets and adapted for food.
Integration capabilities are crucial. Your QA system should connect with inventory management, production scheduling, and supplier portals. Isolated systems create data silos that undermine traceability and force you into manual data entry across multiple platforms.
User adoption determines success. The most feature-rich platform fails if your team won't use it. Prioritize:
- Intuitive interfaces
- Mobile accessibility for floor-level data entry
- Workflows that match how your team actually works—not how software developers think they should work
Scalability matters. The system should handle increasing data volumes, additional facilities, and expanding supplier networks without performance degradation or requiring costly upgrades every few years.
Vendor support makes all the difference. Implementation is just the beginning. You need ongoing assistance with updates, troubleshooting, and optimization as your needs evolve. Evaluate the vendor's reputation for customer support before you commit.
Preparing Your Team for the 2026 QA Landscape
Training and Change Management Strategies
Let's be clear about something important: technology alone doesn't transform quality systems. People do.
Successfully transitioning to modern QA practices requires thoughtful change management and comprehensive training—not just a software rollout and a hope that everyone figures it out.
Start by communicating the "why." Help your team understand that these new systems and processes aren't about making their jobs harder. They're about making compliance easier, reducing tedious manual work, and providing better tools for the important work they're already doing.
See Getting CEOs On Board with Food Safety & Quality Assurance for insights on winning leadership support. Or watch our podcast, Building a Food Safety Culture in Your Organization.
Identify champions within your organization. These are team members who embrace new technology quickly and can help their colleagues navigate changes. Champions provide peer support that's often more effective than top-down training because they speak the same language and understand the real daily challenges.
Provide role-specific training. A line supervisor needs different knowledge than a QA manager, who needs different skills than a document control specialist. Tailor training to what each role actually needs to know. Don't waste a production supervisor's time teaching them document control functions they'll never use.
Implement changes gradually when possible. Rather than overhauling everything simultaneously, roll out new systems in phases. This allows teams to build confidence and competency before taking on additional changes. Success breeds more success.
Gather feedback continuously. Your team will identify issues and improvement opportunities that weren't apparent during planning. Create real channels for input and demonstrate responsiveness by actually acting on practical suggestions. Nothing kills adoption faster than asking for feedback and then ignoring it.
Celebrate wins along the way. When the new system helps you pass an audit more easily, when digital forms save significant time, or when predictive analytics prevent an issue—recognize these successes publicly. Positive reinforcement encourages continued adoption and engagement far more effectively than mandates.
Conclusion: Embracing Continuous, Connected Quality Assurance
Food industry quality assurance is undergoing its most significant transformation in decades. What's happening in 2026 isn't just about new regulatory requirements or trendy technology—it represents a fundamental shift in what quality management actually means.
The era of periodic audits, paper-based documentation, and reactive quality control is ending. It's being replaced by continuous verification, real-time data, and predictive prevention. This evolution demands new tools, new skills, and honestly, new mindsets from everyone in the food safety ecosystem.
For QA leaders, these changes present both challenges and genuine opportunities. Yes, modernizing systems and processes takes work and commitment. But the payoff is substantial:
- Reduced audit preparation time
- Fewer compliance findings
- Faster response to quality issues
- Greater confidence in your quality system's actual effectiveness
The manufacturers who will thrive in this landscape are those embracing these changes proactively—not waiting until compliance forces their hand. They're digitizing their quality systems now, building supplier partnerships based on continuous collaboration, and implementing predictive analytics that turn quality data into actionable intelligence.
Technology platforms like Allera exist specifically to help food manufacturers navigate this transition. By automating supplier management, linking training to procedures, enabling real-time QA data capture, providing AI-assisted compliance checking, and delivering predictive analytics, these systems make modern quality management accessible and achievable—not just theoretically possible.
The question isn't whether your organization will adapt to these changes. Regulatory requirements and competitive pressures guarantee that you will.
The real question is whether you'll lead this transition or simply react to it. Whether you'll shape these changes to your advantage or scramble to meet external demands at the last minute.
Start today.
Take an honest look at where your current quality system falls short of 2026 expectations. Identify your highest-priority gaps. Then take concrete steps toward a more connected, continuous, and data-driven approach to quality assurance.
Your customers, your auditors, and honestly, your team will thank you.
FAQs
What are the 5 functions of quality assurance?
The five main functions of quality assurance in the food industry include:
Process control – Ensuring every production step meets set standards.
Inspection and testing – Checking raw materials, in-process items, and finished products.
Documentation management – Maintaining accurate records for traceability and audits.
Training and education – Ensuring employees understand and follow food safety systems.
Continuous improvement – Identifying weaknesses and optimizing systems for better quality and compliance.
How do I write a quality assurance plan?
To write a Quality Assurance Plan (QAP):
Define objectives: Explain what your QA system aims to achieve.
Outline responsibilities: List roles for QA staff and production teams.
Describe standards: Include regulatory and customer requirements (FDA, SQF, etc.).
Detail procedures: Document SOPs, HACCP steps, inspection schedules, and testing methods.
Set verification steps: Explain how compliance will be monitored and recorded.
Include corrective actions: Describe how non-conformances will be handled.
Review and update regularly – Keep the plan aligned with new regulations and findings.
What is the difference between QAP and QC?
A Quality Assurance Plan (QAP) outlines the processes and systems to prevent defects and ensure consistent quality. Quality Control (QC), on the other hand, focuses on detecting defects in products through testing and inspection. QA is proactive (process-oriented), while QC is reactive (product-oriented).
What is quality assurance in the food industry?
Quality assurance (QA) in the food industry is the process of ensuring that every product meets established safety, regulatory, and quality standards before reaching consumers. It involves setting up systems, procedures, and audits to verify that food production follows Good Manufacturing Practices (GMP), HACCP principles, and customer specifications. The goal is to prevent defects and contamination rather than simply identify them after production.
What is HACCP in quality assurance?
HACCP (Hazard Analysis and Critical Control Points) is a systematic approach used within quality assurance to identify, evaluate, and control potential hazards in food production. It focuses on preventing biological, chemical, and physical risks before they occur. As part of a QA system, HACCP ensures that every step of the production process is monitored and that corrective actions are taken immediately if deviations arise.
What does QA do in the food industry?
QA teams in the food industry develop and enforce policies to maintain consistent food safety and quality. They monitor production lines, conduct inspections, verify sanitation procedures, check supplier compliance, and manage documentation for audits. QA also investigates consumer complaints, trains employees on food safety protocols, and ensures compliance with standards such as FDA, USDA, SQF, or BRCGS.
What are the 5 stages of quality assurance?
The five stages of quality assurance typically include: Planning – Establishing objectives, standards, and procedures; Designing – Creating processes and documentation aligned with standards; Implementation – Training teams and executing QA procedures; Monitoring – Auditing, inspecting, and collecting data to verify compliance; Improvement – Analyzing results and implementing corrective or preventive actions to enhance quality performance.
What are the 5 P's of quality assurance?
The 5 P’s of quality assurance refer to the core elements that influence consistent quality outcomes: People – Trained employees who follow established quality and safety protocols; Processes – Standardized procedures that ensure consistency and compliance; Products – Raw materials and finished goods that meet defined quality standards; Premises – The facility and environment where food is handled and produced; Procedures – Written systems (like SOPs and HACCP plans) that guide safe, repeatable practices.
What is the difference between food safety and quality assurance?
Food safety focuses on protecting consumers from hazards like contamination or illness, while quality assurance ensures that food products meet defined standards of consistency, taste, appearance, and performance. In simple terms, food safety prevents harm, and quality assurance builds trust through reliability and compliance.
.avif)






.avif)

.avif)